2019 BioPacific Conference Sponsor Exhibitor Price and Benefits
Date: June 22, 2019 @ 9:00 am – @ 6:00 pm
Location: San Mateo Marriott, 1770 South Amphlett Blvd, San Mateo, CA 94402

HIGHLIGHTS OF UPCOMING 2019 BIOPACIFIC CONFERENCE
- Winner of 2019 CABS K. Fong Award in Life Sciences
- This full-day event is expected to attract more than 600 attendees of life science professionals from the US, China and other regions
- Opportunities to connect with potential clients for your business
- Cutting-edge science and technology in life sciences including cancer immunotherapy, cell therapy, precision medicine, and medical technology
- Success stories of entrepreneurs and emerging companies in US and Asian Pacific Rim
- Patent and legal considerations in life sciences
- Latest biopharmaceutical development policies and regulations
- Cross-border investment and M&A in life sciences
- BioPartnering Forum to facilitate interactions including project introduction and talent recruitment
BioPacific Conference is the annual flagship event of the CABS. Our mission is to bring scientists, executives and biopharmaceutical professionals from the Pacific Rim countries together to discuss the latest advances in the world of life sciences, the unprecedented challenges and promises facing the industry, and the breathtaking developments and opportunities in Asia/Pacific countries.
Featured Podium Talks
Keynote: Biomarkers and translational research in cancer therapy
Ron Mazumder,PhD, VP, Global Head of Oncology Biomarker,Genentech
Dr.Ron Mazumder has more than 20 years experiences in predictive biomarkers and precision medicines. He has served senior positions at J&J and Merck before joining Genentech in 2016. His talk will focus on predictive biomarker and translational research in immuno-oncology.
Talazoparib: From Virtual Drug Discovery to FDA Approval
Leonard Post, PhD, CSO, Vivace Therapeutics
This is a legendary drug discovery story you cannot miss. Talazoparib, a novel PARP inhibitor, was initially discovered in 2007 by a US-based virtual company Lead Therapeutics, with most of its discovery work done in China. Dr. Leonard Post, CSO of Lead Therapeutics, was involved in its discovery. In 2010, BioMarin acquired Talazoparib for $97M and Dr. Post became the CSO of BioMarin. In 2015, Medivation acquired Tapazoparib for $570M and in 2016, Pfizer acquired Medivation. In 2018, Talazoparib was approved for breast cancer. Journey through Talapzoparib for past 12 years, Dr. Leonard Post will share his first-hand experiences from building a virtual company to FDAapproval.
CABS 2019 K.Fong Award Speech- The Future of the Biopharma Industry: Innovation Beyond Science
John V. Oyler, Founder, CEO and Chairman, BeiGene
During a dinner in 2010, John Oyler and renowned scientist Dr. Xiaodong Wang decided to “build China’s Genentech” and thus BeiGene was born. Within a decade, John has ledBeiGene to be one of China’s most innovative biotech companies. BeiGene is listed in both NASDAQ and HKSE with market capital more than $7B. What are the stories behindBeiGene’s 10 years growth? What is the current status and future of biopharma industry, both in China and in the world? John will share his perspective during CABS 2019 K. Fong award speech. After the speech, Dr. K. Fong and John Oyler, a legendary venture investor and a legendary entrepreneur, will have a face-to-face chat to discuss more on China’s biopharma industry.
New Approaches To Neurodegenerative Disease Drug Discovery and Development
Zachary Sweeney, PhD, Head of Therapeutic Discovery, Denali Therapeutics
Alzheimer’s disease is one of the most difficult diseases in biopharma drug R&D. From 1998 to 2017, there are >100 failed trials for it. Founded by a group of Ex-Genentech veterans in neurosciences and fueled with record-setting Series A funding, Denali believes it can tackle Alzheimer’s disease differently. What are the unique approaches? Before joining Denali, Dr. Zach Sweeney has been senior leaders in Novartis, Genentech and Roche. He will present an overview of selected development programs of Denali.
Key Developments: CFIUS, Export Control Reform, and Recent Decisions Affecting Life Sciences Investment and Licensing
Joseph Benkert, Senior Advisor, National Security Group, Morrison & Foerster
Last summer the Committee on Foreign Investment in the United States (CFIUS) issued new regulations on the review of inbound foreign investments and export control of outbound licensing. What are the impacts of these new regulations on US – China cross-border investment? How to navigate these new changes? Joseph Benkert is a former US Department of Defense Assistant Secretary of Defense for Global Security Affairs under both the Bush and Obama administrations. He has many years experiences handling complex matters before CFIUS. Joseph has presented the latest CFIUS regulations at CABS 2019 Investor Forum in January 2019. He is back to CABS with new updates on CFIUS regulations since January.
Applying Precision Medicine One Patient at a Time
Shivaani Kummar, MD, Professor of Medicine, Stanford University
With the increasing number of anticancer therapeutic choices, it is possible to tailor treatment options based on individual patients. Professor Shivaani Kummar is an internationally renowned expert in cancer therapy clinical trials, especially in early stage trials. She is currently the Director of the Phase I Clinical Research and Translational Oncology Programs at Stanford University, where she has managed ~20 clinical trials in oncology. In this talk, Professor Kummar will focus on using predictive biomarker and genomic drivers to identify the “right” patients and how to design better early clinical trials that can help making key decisions on later stage trials.
| New Diagnostics Ecosystem to Accelerate Drug Discovery and Medical Innovation |
|
Jason Liu, PhD, MBA, CEO, WuXi Diagnostics The increasing cancer incidence and mortality accelerate demand for targeted drugs and companion diagnostics and economic pressures to develop maximally efficient treatments in China. With the encouragement from the China government, it’s estimated that by 2021, the market size of companion diagnostics will reach 741 million USD, with CAGR of 28%, far exceeding the global scale as 20.1%. WuXi Diagnostics enables precision medicine through a unique dual-enabling platform for in-vitro diagnostic service and product innovation. Our advanced platform integrates multi-dimensional, multi-omics medical data to generate deep clinical insights, and pioneer innovation by building a novel eco-system for healthcare industry. |
Featured Panel Discussion
|
Panel Discussion: innovative therapy in oncology, what is next? |
|
Moderator: Cheni Kwok, PhD, CLP, Managing Partner and Founder, Linear Dreams |
|
Panelists: |
|
Wenfeng Xu, PhD, VP, Henlius |
|
Jean Cui, PhD, CSO, Turning Point Therapeutics |
|
Mark Nevins, MS, VP of Business Development, Apexigen |
|
Peiwen Yu, PhD, VP of Discovery Biology, Exelixis |
|
Tara Arvedson, PhD, Director of Research, Amgen |
| Yanyan Zheng, PhD, Principle Scientist, Merck |
|
|
In this panel discussion, experts from both large biopharma companies such as Amgen and Merck, as well as rising-star oncology startups, will discuss what's next wave of anticancer drugs.
Key Insights from AACR and ASCO 2019
- What was the most exciting data at AACR or ASCO this year?
- What are the emerging hot targets?
Where is the field going in terms of next generation biotherapeutics?
- Antibody formats (ADC, bispecific, multi-specific...)
- New fusion proteins
- Cell therapies (such as CAR T cell, stem cells...)
- Gene therapies (virus-based, modified RNA, anti-sense... )
- Cancer vaccines
What are the future trends for small molecule cancer drugs ?
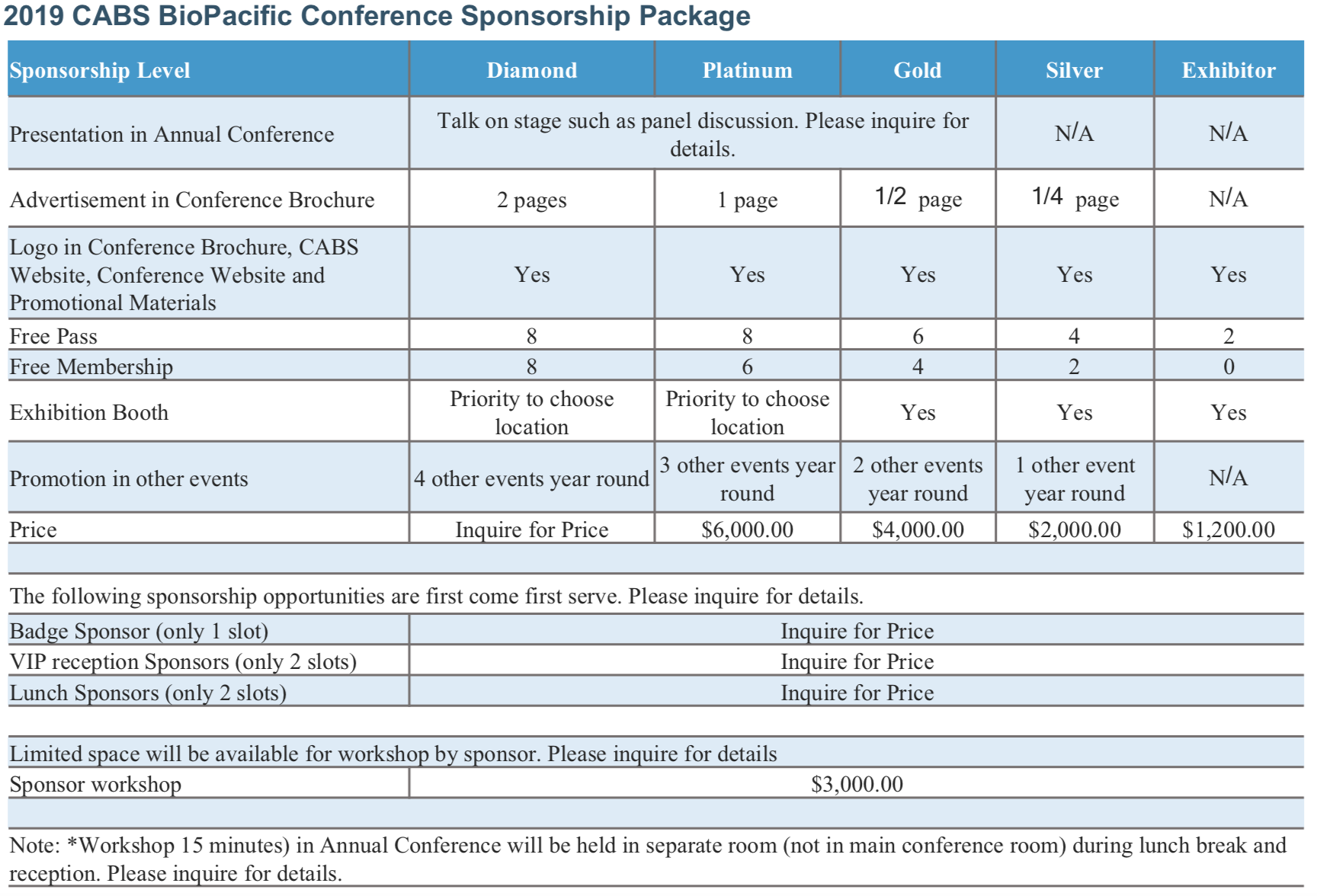
Welcome 2019 Sponsors!
As of May 27, 2019, all exhibition tables for 2019 BioPacific Conference have been reserved by sponsors. If you have not received invoices from CABS, please do not pay online directly. You are very welcome to contact fundraising@cabsweb.org if you still wish to sponsor CABS without exhibition table. Thank you very much!

2018-2019 CABS Sponsorship Opportunities & Benefits:
- Year round sponsorship opportunities including the highly anticipated BioPacific Conference
- Leverage our extensive network of life sciences professionals in the U.S. and Pacific Rim Countries
- Receive US tax benefits for your financial contribution
.
