Replay Available: The Race of COVID-19 Vaccine
Date: May 16, 2020 @ 8:00 am – July 16, 2020 @ 8:00 am
Location: Online
On May 16, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled
“Fireside Chat with Dr. John Wang: The Race for COVID-19 Vaccines”.
By Dr. John Wang, CEO of ImmuOn Therapeutics
A replay of the webinar can be accessed here

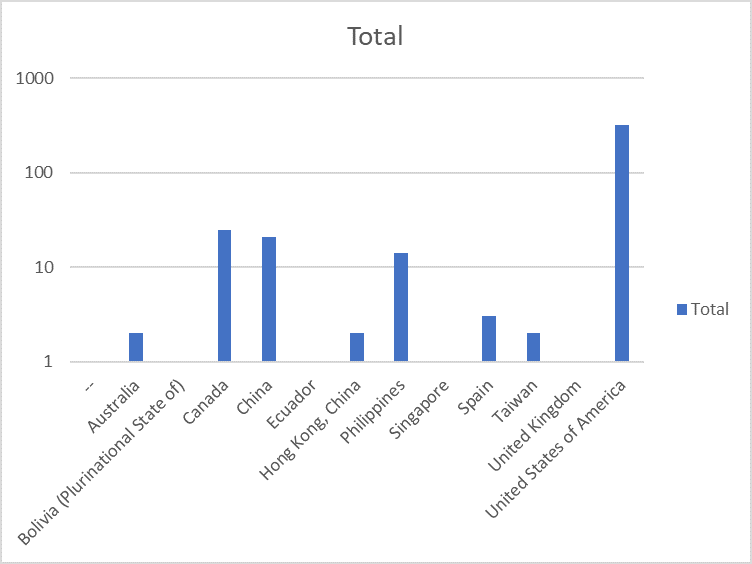
The webinar attracted almost 300 attendees from around the world
(see attendees distribution below)
Dr. John Wang started his research career at the Department of Molecular Immunology of the Commonwealth Serum Laboratories (CSL), Australia and then its spin-off company "Mimotopes". He joined US company Chiron in 1995 and then Novartis in 2006, as Associate Director of Drug Discovery.
John returned to China and founded ImmuOn Therapeutics in 2012 to focus at research and development of therapeutic vaccines for chronic infection and cancer. In 2018 the cancer vaccine business was spun-off to become IOVaxis Therapeutics. The company currently has two cancer vaccine IND applications waiting for approval by NMPA. Since 2014, John has been invited by the China Medical Economic News to be the columnist for the column "Immune, Gene and Health". He has so far published over 200 articles.
While in US, John joined the International Society for Biomolecular Screening (SBS) In 1996, and served as the Chair for both regional and International Annual Conferences, and later two terms of its Board member and President-elect before the society merged into the Society of Laboratory Automation and Screening (SLAS). In 1998, Dr. Wang also co-founded the Chinese-American BioPharmaceutical Society (CABS).
Dr. Yang Tian, President of CABS, started the webinar with an introduction of CABS and its efforts for combating COVID-19, including a series of scientific webinars and a PPE donation campaign.
Dr. Wang then started his talk on why we need COVID-19 vaccines, followed by a review of five major vaccine technology platforms and current major players in the field. Then Dr. Wang discussed clinical trials of COVID-19 vaccine, specifically the phase 3 challenge trail. Further on, Dr. Wang talked about potential risk factors of developing a COVID-19 vaccine, including ADE. In the end, Dr. Wang discussed current status of COVID-19 diagnostics and epidemiology features. In the end, Dr. Wang expressed his optimism on developing an safe and effective COVID-19 vaccine based on current technology we have.
There were 3 poll surveys from the audience during the webinar.
The first survey question was “which vaccine technology will likely give the 1st COVID-19 vaccine approved?”
l 31% of attendees chose “inactivated or attenuated viruses”,
l 28% of attendees choose “mRNA vaccines”.
The 2nd survey question was “when will the 1st COVID-19 vaccine be likely approved?”.
l 45% of attendees chose by June 2021,
l 36% of attendees chose byDecember, 2020.
The 3rd survey question was “would you take the COVID-19 vaccine if it is approved and available?
l 65% of attendees chose “yes”
l 35% of attendees chose “No”.
We would like to thank Dr. John Wang for his time and efforts for this webinar. We also want to thank Jenen Tan, Hesong Han, Guanghui Han, Carrie Wang, for organizing the logistics of the event and thanks to Zoom and Beijing Well-being Foundation for providing technology support for this webinar.
To learn more about CABS’ effort in the COVID-19 response, please visit our website www.cabsweb.org.
A replay of the webinar recording will be available on this site.
.
