We are thrilled to announce that CABS will host the 2024 BioPacific Conference (The 24th Annual Conference of CABS) on October 28th, 2024, at the South San Francisco Convention Center. This year is significant as it marks the 26th anniversary of CABS. We look forward to welcoming you to this exciting conference and celebrating our quarter-century milestone together.
www.cabsweb.org; www.biopacificconference.org;
For sponsorship opportunities: fundraising@cabsweb.org


South San Francisco Convention Center
Introduction:
What does it take to bring clinical evidence to obtain market approval? This workshop is designed to gain a “big picture” of the entire drug development process from IND to NDA/BLA. It will highlight regulatory requirements, the framework for robust study design, medical monitoring and benefit & risk assessment, and elements of clinical operations and CRO program management. Key principles and concepts will be illustrated with case studies. This workshop will provide an opportunity for CABS members who are interested in expanding professional networks to clinical development for career development, industry partnerships or strategic collaborations.
Presentation Titles and Speakers:
1. Presentation Title: Brief introduction of clinical development
Speaker: Wei Dong, MD, PhD, VP and Head of Drug Safety at Denali, 2010-2011 CABS President
2. Presentation Title: Regulatory overview: the journey from IND to NDA/BLA including case examples
Speaker: Wen Liu, PhD, VP of Regulatory Affairs, Lyell Immunopharma
3. Presentation Title: Statistical considerations in clinical trial designs
Speaker: Jing Yi, PhD, Head of Data Science and Global Development Team Leader, CARGO Therapeutics
4. Presentation Title: Clinical operations and CRO program management
Speaker: Angela Wang, Commercial Director at Novotech
5. Presentation Title: Clinical Monitoring Priorities and Responsibilities
Speaker: Harry Liu, MD, MPh, MBA, Executive Director, Clinical Development, ALX Oncology
6. Presentation Title: The science and practice of drug safety
Speaker: Wei Dong, MD, PhD, VP and Head of Drug Safety at Denali, 2010-2011 CABS President
List of Speakers:
Wei Dong, MD, PhD
VP and Head of Drug Safety at Denali
2010-2011 CABS President
Wen Liu, PhD, VP of Regulatory Affairs, Lyell Immunopharma
Jing Yi, PhD, Head of Data Science and Global Development Team Leader, CARGO Therapeutics
Harry Liu, MD, MPh, MBA, Executive Director, Clinical Development, ALX Oncology
Angela Wang, Commercial Director at Novotech,
Organizing committee:
Wei Dong, MD, PhD, VP and Head of Drug Safety at Denali, 2010-2011 CABS President
Liping Meng, PhD, Senior Scientist II at Gilead Sciences, Co-chair of CABS Science & Technology Committee
Yan Wang, PhD, Director at ChemPartner, Co-chair of CABS Science & Technology Committee
Jessica Sun, PhD, Executive Director, In Vivo Pharmacology at Terremoto Biosciences, 2024-2025 CABS President
Exclusive Sponsor:
NovoTech
Bio of Speaker:
Wei Dong is the VP and Head of Drug Safety at Denali Therapeutics where she builds and leads safety and pharmacovigilance organization. She oversees the scientific, operational and product strategy aspects of global safety during clinical development and prepare the organization as a whole to address future marketing authorization applications and multinational postmarketing safety requirements. Wei brings extensive R&D experience in global drug development to improve patient care. She held executive leadership roles in Genentech and Roche in the areas of drug safety, epidemiology and Asia Pacific product development contributing to the approval of multiple breakthrough medicines. Most recently she served as the VP and Head of Safety at Corcept Therapeutics building up its safety team and ensuring successful regulatory inspections. Wei obtained her MD from Shanghai Medical University and PhD in Epidemiology from the London School of Hygiene & Tropical Medicine, University of London. She lectured at UCL Medical School, U.K., and was an Assistant Professor of Epidemiology and Community Medicine at Mount Sinai School of Medicine before joining the industry. Wei is a long-term supporter of the CABS vision and community and has served as the CABS Present 2010-2011. )
Jing Yi is the Head of Data Science, Global Development Team Leader for CRG-022 at CARGO Therapeutics since May 2023. Jing has over 17 years of industry experience with a proven record in oncology clinical development and medical affairs, and in functional and cross-functional leadership.
Jing has been involved in many successful global regulatory interactions and approvals, including Avastin across various tumor types. She has built and led multiple high performing cross-functional teams and initiatives. Prior to joining CARGO, Jing spent over 2 years at Erasca as the Senior VP of Data Science and PTL of ERAS-601 program. Jing was the Global Development Team Leader for Tecentriq at Roche/Genentech prior to joining Erasca, where she led a cross functional team and was accountable for the overarching strategy, development and implementation of cancer immunotherapy in Lung and Head/Neck Cancers. She also managed a group of medical directors and clinical scientists. Prior to her cross-functional leadership role, she was the biometrics lead of multiple molecules and managed a group of statisticians who supported many pivotal studies at Roche/Genentech. Jing received her PhD. in Statistics from the University of California, Berkeley.
Angela Wang has extensive business development, investment, product management, and research experience in the biotech industry. As the China Commercial Director at Novotech, her focus is to bridge the needs of biopharma companies in North America/Europe with China. This involves guiding clients through regulatory, clinical, and commercialization strategies.
Angela previously held Sr. Director of Business Development position at ClinChoice, Director of Investments and Operations at several venture capital firms as well as various senior product management and marketing roles at ThermoGenesis, Thermo Fisher Scientific, and Abaxis.
Angela received her MBA from Rensselaer Polytechnic Institute and B.S. in Cell Biology from Wuhan University. Angela is based in the San Francisco Bay Area.
Wen Liu is a seasoned regulatory affairs professional in biotech. She has been serving as the VP of regulatory affairs at Lyell immunopharma since 2021, a clinical stage cell therapy company with genetic reprogramming technologies for oncology. Prior to joining Lyell, she joined Genentech in 1998 as a scientist in research after acquiring her PhD. In 2004, she transitioned to clinical regulatory affairs and over time has established as a leader in regulatory strategy and filings for groundbreaking oncology therapies. At Genentech/Roche, she was instrumental in the successful development and approval of major oncology therapies worldwide as a global regulatory affairs leader. At Lyell, she continues to drive regulatory excellence to bring transformative therapies to clinic.
Harry Liu is currently an Executive Director Clinical Development in ALX Oncology, where he is the clinical lead for the head and neck cancer programs. Prior to ALX Oncology, he had worked in Genentech and Amgen, and taken leadership roles in clinical development and clinical safety functions. Harry received his medical degree from Norman Bethune University of Medical Sciences, MPH in Epidemiology from UC Berkeley, and MBA from UCLA Anderson school.
Lunch will be provided. Limited seats. RSVP is required. Please register as early as possible.
1633 Old Bayshore Hwy #280, Burlingame, CA 94010
Please note the address has been updated to 3000 El Camino Real, Building # 5, Suite 125, Palo Alto, CA 94306.
******************************************
Call for all innovators and entrepreneurs who are looking to turn their ideas to startups!
Present your ideas to seasoned VCs, veteran BDs, and your potential co-founders– get feedbacks, funding opportunities as well as the tickets to present at CABS flagship events such as annual BioPacific Conference or Investor Forum during JPM.
Whether you are at seed run or series A/B, all stages are welcomed!
Demo day agenda:
Road show 2.30-6.30 pm, networking reception 6.30-8.00 pm
Agenda:
2.30–2.45: Check-in and social
2.45-2.55: Open remark by CABS president Dr. Jessica Sun
2.55-3: Welcome greetings by K&L (Special thanks to K&L as our exclusive event sponsor )
3–4: First Company Presentation Session (10 min presentation + 5 min Q&A)
Amberstone
Ariz
Coastar Therapeutics
DeepSeq.AI
4–4.40: First panel
4.45–5.45: Second Company Presentation Session
ReRX
Spect
SunnyBay
Synlico
5.50–6.30: Second panel
Networking reception: 6:30–8.00
Opportunity to showcase your idea in front of VCs, BDs, seasoned and first time entrepreneurs, as well as scientists with similar passion. Be ready to mix and mingle with your potential partners!
For general in-person attendance, please register at link below.
For any questions, please feel free to contact CABS at lu.lu@cabsweb.org, Lu Lu

3000 El Camino Real, Building # 5, Suite 125, Palo Alto, CA 94306
Since its inception in 2010, the Career Advisory Network (CAN) Program has evolved into a cornerstone event of the annual calendar for the Chinese American Biopharmaceutical Society (CABS). Orchestrated by the Business and Career Development (BCD) committee, CAN stands as a pivotal mentoring initiative that facilitates personalized, one-on-one mentor-mentee pairings. The program is designed to provide tailored guidance to participants, assisting them in navigating the intricacies of their professional journeys. This includes support in areas such as business development, entrepreneurship, and the pursuit of scientific careers within both academia and the private sector. On April 13, 2024, the CABS CAN program proudly launched its latest iteration with a kick off meeting at the Hanhai Silicon Valley Center, marking the beginning of another year of transformative mentorship and growth.
Jessica Sun, the 2024 CABS president, gave a brief introduction of CABS and kicked off the meeting .
Followed by a very insightful introduction from the event Sponsor - Dr. Sheng Wang from Zelixir. Zelixir is a company focused on AI-driven synthetic biology and intelligent manufacturing. By utilizing exceptional AI-driven bioengineering technology, it combines AI with synthetic biology to lead the future of extraordinary biotechnology.
Mr. Tao Huang, President and CEO of NeuCyte, served as an esteemed mentor representative. He recounted his professional journey, imparting wisdom from his own career development. Additionally, Mr. Huang offered essential guidance to the mentees on the art of effective communication with their mentors and provided strategies for attaining success in their careers.
Additionally, Professor Ruben Luo, the Assistant Professor of Pathology at Stanford University as well as the Associated Director of Clinical Chemistry Laboratory at Stanford Health Care, a fellow mentor, imparted his wisdom by sharing his personal experiences and providing insightful guidance.
Following the sharing session, mentors and mentees actively engaged with one another, reinforcing the relationships and forging new bonds.
The kickoff meeting marked a stellar beginning for the thrilling CAN initiative, leaving participants infused with a sense of empowerment and a strong motivation to drive success. We extend our heartfelt gratitude to Ava Song, Jonathan Wong, and Le Cai for their exceptional coordination efforts in launching this program.
We thank again for our exclusive sponsorship from Zelixir. More information about our sponsor can be found here: https://www.zelixir.com/
Hanhai Biolabs---1633 Old Bayshore Hwy STE 280, Burlingame, CA 94010
The Chinese American Biopharmaceutical Society (CABS) is thrilled to announce a new summer internship program focused on the cutting-edge field of data science applied to drug discovery and development.
As our industry generates unprecedented amounts of data from genomics, proteomics, clinical trials and real-world evidence, there is a growing need for talented data scientists who can derive insights to drive innovation. CABS is committed to helping bridge this talent gap and train the next generation of data leaders in biopharma.
This 10-week non-paid internship program will provide an immersive experience for undergraduate and graduate students to work alongside data science teams at top CABS member companies in the San Francisco Bay Area. Interns will get hands-on experience applying the latest machine learning, AI and data analytics tools to solve real-world challenges across all phases of the drug development lifecycle.
Not only will interns gain technical data skills, but they will also learn about the biopharma business through workshops, networking events, and mentorship opportunities with CABS member company leaders, entrepreneurs and investors.
A primary focus will be nurturing a talented and diverse pipeline of data science professionals, including those from Asian American and Pacific Islander backgrounds. In addition to students from top universities like UC Berkeley, Stanford and UCSF, we strongly encourage applications from students at universities across the Pacific Rim and Asia.
Internship Timeline:
- Applications Open: April 1, 2024
- Application Deadline: June 5th, 2024
- Internship Dates: June 15 - August 25, 2024
This internship program represents an incredible professional development opportunity. We invite all eligible students passionate about life sciences and data to apply!
For more details on the program, eligibility requirements and how to apply, please contact:
Please click this link or scan the QR code below
to finish the online application!

We look forward to receiving applications from the world's brightest data science minds!
Sincerely,
President, CABS
April, 2024
CABS, a certifying organization of President’s Volunteer Service Award (PVSA)
On March 6, 2024, Chinese American Bio/Pharmaceutical Society (CABS) was officially approved as a certifying organization empowered to confer the President’s Volunteer Service Award (PVSA). CABS will be responsible for verifying and certifying that volunteers have fulfilled the necessary requirements to qualify for the PVSA within a 12-month period (e.g., from February 16, 2024, to February 15, 2025).
Any eligible adult or student volunteer who has contributed to CABS events will be required to self-track their hours on the service log sheet. The co-chairs of each committee will oversee the review and verification process. It is imperative that volunteers diligently maintain their hours tracking records as proof during potential audits. Please use the “PVSA Service Log” as a template.
For any inquiries or clarifications, please reach out to BCD Co-chair: Lin Wang, PhD; lin.wang@cabsweb.org

Please mark your calendar, and your early online registration will be greatly appreciated. We look forward to seeing you there and celebrating Year of the Dragon together!
Thanks to our sponsors(see more detail in the end):

Thanks to our sponsors:

Contact: David Ri Sun CPA&MBA, License#123434
Cell: 408-6610087
Address: 97 E. Brokaw Rd, Suite 310-F, San Jose, CA, 95112
Email: david@apobridge.com
Website: www.apobridge.com

Contact: Winny Lin MBA, LUTCF Financial Planner License#0E31564
Cell: 415-309-7300
Address: 951 Mariner Island Blvd, Suite 600, San Mateo, CA, 94404
Email: wylin@ft.newyorklife.com
Website: www.callwinny.com
%20PNG_HR.png)
GenScript is a leading provider of advanced life sciences solutions, empowering researchers worldwide to achieve breakthroughs in their scientific endeavors. With expertise in custom Gene Synthesis, Protein Expression, CRISPR Technology, Reagent Antibody Development, and Virus Packaging, GenScript offers precision-engineered tools and services to accelerate research in Genomics, Gene Cell Therapy, and Antibody-Drug Discovery. Known for reliability and innovation, GenScript is a trusted partner for scientists seeking high-quality, customized solutions to propel their experiments and discoveries forward. Explore the potential of your research with GenScript at www.genscript.com.
Name: Fiona Wu - Sales Account Manager
Cell: 732-902-1603
Email: fiona.wu@genscript.com, Website: www.genscript.com
Contact: Xingyu Shen, CEO,PhD
Cell: 510-570-9504,
Address: 1257 Magnolia Ave, San Carlos.
Email: Connect@quanmol.com
Website: www.QuanMol.com


Foster City, William E. Walker Recreation Center, 650 Shell Blvd. Foster City, CA 94404
Lunar New Year is the most important holiday celebrated by Chinese and other Asian communities globally. On February 4th, 2024, despite having the worst storm in a year in the San Francisco Bay Area, more than 250 members and friends of Chinese American Bio/Pharmaceutical Society (CABS) gathered at the Foster City Recreation Center to celebrate the beginning of the Year of Dragon. As a tradition of CABS, this gala was hosted by its Social Life Committee, featuring a wide variety of spectacular live cultural performances, traditional Chinese cuisine, and auspicious wishes.
The attendees were first greeted with vibrant decorations of red lanterns, hand-written couplets and many other festive ornaments as they entered the venue. The gala was then kicked off and livened up by the rhythmic beats and energetic movement of a traditional Chinese waist drum folk dance, symbolizing the expression of joy, luck, unity and prosperity for the new year.
There were 20 different performances at the Gala, including classical folk dances, contemporary dance, Jazz, Latin and break dances, martial arts, singings, qipao runway show, traditional Chinese stand-up comedy, as well as both modern and classical string instrument performances. The program was graciously delivered by more than 100 performers from the Bay Area, showcasing the generosity and talents of the community, as well as the rich heritage and diversity in Chinese culture.
While indulging in the dazzling performances, attendees were served with authentic Chinese dishes that symbolizes longevity, abundance, and fortune according to the Chinese Spring Festival traditions. Throughout the event, attendees were highly engaged in cultural activities such as calligraphy, learning different forms of Chinese characters and bringing home their favorite couplets expressing best wishes. At the other side of the venue, attendees were provided with opportunities to meet with our sponsors, learn about their products and services, and receive the unique giveaways.
During intermissions, 10 New Year gifts, including two grand prizes of gift card, were given out to winners from three rounds of “Lucky Draw”. The winners were picked out by the two co-chairs of the Social Life Committee, Ms. Li Wang and Ms. Katherine Guan, and distinguished guests including sponsors and the founding president of CABS, Dr. Keting Chu.
Whether it’s a festive gala, a cultural celebration or a networking event, the CABS Chinese New Year Gala provided a dynamic platform for attendees to explore the roots of Chinese culture with their family and friends, while expanding their professional connections with new friends.
CABS would like to extend its sincere appreciation to all volunteers, members, and participants for their support to make this event a huge success. A special shout-out to Li Wang, who dedicated tremendous time and effort to make this event possible. This event was sponsored by Apobridge CPA Firm, GenScript, HungryPanda, New York Life Insurance and QuanMol Tech. Thank you all for your support and we wish you joy, prosperity and success in the Year of the Dragon! Hope to see you again next year!


-
CABS is pleased to announce the awardees of the 2023 CABS Award
-
-
Congratulations to all the award winners for their outstanding achievements and contributions! Your dedication, hard work, and talent have been recognized and celebrated by all of us at CABS. We are truly grateful for your remarkable efforts, which have inspired and uplifted everyone around you. Thank you for your unwavering commitment to excellence and for being an inspiration to us all. We extend our heartfelt congratulations and best wishes for your continued success and accomplishments in the future.
-

-
This year’s annual Chinese American Bio/Pharmaceutical Society (CABS) Executive Council Transition Meeting convened on January 27th, 2024, in Burlingame. Attendees included esteemed members from both the 2023 and 2024 Executive Committees.
Setting the tone for the gathering, Dr. Yuying “Kate” You, the immediate past CABS president, artfully delineated the remarkable achievements of the year of 2023. These notable accomplishments encompassed a spectrum of successes, featuring events such as the 2023 Investor Forum, 2023 BioPacific Conference, the 25th Year Anniversary Reception, and activities as well as workshops organized by the Social Life committee, International Collaboration Committee, and Science & Technology Committee.
Speaking on behalf of the Board of Directors, Dr. Yang Tian, the former president of CABS, took the stage to elucidate the meticulous bylaws and code of conduct that guide the organization.
Dr. Jessica Sun, President-Elct of CABS 2023 and Chair of the 2023 BioPacific Conference Organizing Committee, engaged the audience with a comprehensive review of the preceding year. They shared both the triumphs and the invaluable lessons learned, casting an optimistic gaze toward the promising future of their respective committees in the upcoming year.
The transition from the 2023 EC to the 2024 EC was formally concluded as Dr. Yuying “Kate” You, the president of CABS 2023, ceremoniously passed the President’s Cup, signifying the CABS Presidency, to Dr. Jessica Sun, the newly inaugurated CABS president for 2024.
Jessica then announced the members of the 2024 CABS EC listed below:
Finally, Kay Tong, the newly elected President-Elect and Chair of the 2024 BioPacific Conference, provided insights into how the new EC will strategically prepare for the Conference.
Please join us to thank the 2022-2023 CABS EC for their excellent service and congratulate the 2024 CABS EC and wish them great success!
Hanhai Biolabs
The “Road Map for Transnational Planning” workshop hosted by the Social Life Committee (SLC) of CABS on January 20th attracted more than 40 attendees at Hanhai Biolabs in Millbrae, CA. This in-person event discussed strategic plannings designed for successful professionals and business owners to minimize tax while maximizing their foreign assets to help achieve financials goals for their family and future generations.
The workshop kicked off with our first speaker, Mr. Frank Huang, J.D. LL.M, Corporate VP and Advanced Planning Consultant, who has over 20 years of experience in estate planning, trust administration and business succession planning. During his talk, Mr. Huang elucidated the issues and process of probate in California with and without a Will. He also introduced strategic ways to avoid probate for the benefits of time, cost, and privacy.
Our second speaker, Mr. Tony Li, Corporate VP and Advanced Planning Consultant, discussed the upcoming changes in the federal tax system and the reporting requirements of foreign estates for U.S. citizens and residents, highlighting the benefits of leveraging a team of experienced financial professionals and attorneys for designing personalized financial plans.
Finally, Ms. Winny Lin, MBA, LUTCF, Financial Planner and Investment Advisor, shared two case scenarios in which her high-income clients successfully retain legacy control over their multi-million foreign assets while minimizing tax liabilities for their heirs and beneficiaries through advanced estate and trust planning.
Speakers received many follow-up questions from the highly engaged audience during the workshop, reflecting widespread of interest on the topics being discussed. This event marks another success on CABS’s commitment to our mission of serving the community.
(Organizer: Social Life Committee (SLC); Editor: Katherine Guan; Photographers: Li Wang, Kay Tong; Supporting Committee: Public Relation Committee (PRC))
Hanhai Biolabs
CABS 2024 Executive Council Election is Open!
Please Cast Your Votes!
You are invited to elect CABS 2024 EC team.
Click the following link to cast your vote. You can vote for up to 24 candidates.
https://www.cabsweb.org/2024-ec-election/
Vote by the deadline: 12AM, January 23, 2023 (PST)
online
Free event, registration is required. Please click HERE to register!
Topic: Everyone Needs a Map
You’re invited to a workshop on 1/20/2024 ( Saturday) at 2:00pm to 4:00pm in Burlingame Area on
[ Road Map for Transnational Planning and Assets Protection ]
You are invited to attend the complimentary Road Map for Transnational Planning workshop. During this workshop, we’ll deeply discuss new strategies designed specifically for successful professionals, business owners and affluent people like you, to help minimize your tax while maximizing the assets you pass to your heirs; to help achieve the financial goals you have set for yourself and your family and allow you to build generational wealth!
Topics covered include:
• Transnational and pre or post -immigration planning strategies
• Tax system
• Asset protection
• Estate Taxation and Advanced Trust Planning.
• Additional Reporting Requirement for Foreign Entities.
• Legacy Control
• California probate problems
• Cases analysis
Space is limited, so please register today. I hope you’ll join us for this discussion on some of the most essential strategies that families should consider. Please do not hesitate to reach out with any questions you may have.
Keynote Speakers:
-
Tony Li, Corporate Vice President, Advanced Planning Consultant.
20 years of working experience in financial industry. As a senior specialist, Tony works with high-producing advisors and their affluence clients to develop proprietary financial plans for sophisticated estate planning.
-
Frank Huang, J.D., LL.M. Corporate Vice President, Advanced Planning Consultant.
Over 20 years of significant experience in estate planning, trust administration, and business succession planning.
-
Winny Lin, MBA, LUTCF, Financial Planner, Investment Adviser.
Miss. Lin specializes in retirement and estate planning. As a well experience planner, she has substantial practice working with tax professional and attorneys to help high-net-worth clients to maximize assets protection, reduce tax liabilities, and retain control.
Date: 1/20/2024 ( Saturday Afternoon); Time: 2:00pm – 4:00pm
Location: 1633 Old Bayshore Highway, Burligame, CA 94010.
Phone: 415-510-1291.

Free event, registration is required.
Please click HERE or scan the QR code below to register!

Hanhai, 1633 Old Bayshore Highway, Burligame, CA 94010.
|
CABS 2024 Executive Council Application is Open! |
|
We believe in 'Together, We Make CABS Greater!' Join the CABS 2024 Executive Council (EC) Team and contribute to our shared success. Submit your application here. Application deadline: 12 AM, January 14, 2024 Please note that only applications from active CABS members will be considered. If you are not already a member, be sure to join CABS before submitting your application. As a token of gratitude, once you become an EC member, your membership will be extended for another year. Learn more about who we are and what we do by clicking here: We look forward to welcoming dedicated individuals to our EC team. Together, let's continue to make CABS a thriving community! |
online
Click HERE to register, please!

Click here to download 2024 CABS Investor Forum Program Brochure! .pdf
CABS is a volunteer-based, non-profit organization headquartered in San Francisco, committed to advancing the life sciences and biotech industry through education, collaboration, and networking.
The annual event, Investor Forum, held in conjunction with the JP Morgan Healthcare Conference, has been a hallmark of CABS for nearly 15 years. This year's forum is scheduled to take place on January 10th, 2024, from 8:00 am to 1:30 pm at Morrison Foerster’s San Francisco office (425 Market St, San Francisco, CA 94105). Due to venue capacity restrictions, the forum will have both on-site and online attendance, expecting over 130 on-site participants and more than 100 online attendees.
Our hybrid format will include two insightful panel discussions covering essential topics such as entrepreneurship, investment trends, licensing, and strategic considerations, as well as new opportunities and challenges in life sciences.
INTRODUCTION OF MODERATORS AND PANELISTS
Panel Discussion #1: Reading the Tea Leaves for Biotech Investment in 2024 and beyond
Alex J. Zhang, PhD, CEO, OneTwenty Therapeutics; President of CABS 2017-2018
Dr. Zhang is the Founder and CEO of OneTwenty Therapeutics, Inc., a clinical stage iPSC-based cell therapy company. He was the Chief Scientist of Hanhai Holdings Group, and the CEO of Hanhai Silicon Valley, Inc., an early-stage investor and cross-border incubator for life sciences and deep tech startups based in San Francisco Bay Area. Prior to Hanhai, Alex was the Co-founder and Managing Partner of Enverest, LLC., a Silicon Valley based innovation solutions and investment advisory firm, with branch offices in China and Singapore. Prior to founding Enverest, Dr. Zhang spent over four years at Thermo Fisher Scientific, where he held several senior business roles. From 2001 to 2009, Dr. Zhang was a Senior Scientist at Tularik Inc. (acquired by Amgen in 2004), where he led drug discovery endeavors in oncology, cardiovascular and metabolic diseases therapeutic areas. Over the past decade, Dr. Zhang has been advising a number of successful biotech, MedTech and digital health startups and venture capital firms.
Dr. Zhang is currently a Board Member of the Chinese America BioPharmaceutical Society (CABS). He served a number of leadership roles in the Executive Council of CABS, including as the President in 2017-18. Dr. Zhang earned MBA degree at Cornell University, PhD in Organic and Analytical Chemistry at Texas A&M University, and BS in Chemistry at Shandong University. His research has led to the publication of 17 peer reviewed articles and 4 patents.
Douglas Crawford, PhD, Managing Partner, Mission BioCapital, and General Manager, MBC Biolabs
Doug’s goal is to help entrepreneurial scientists create successful startups. His dream is that every entrepreneurial scientist with a dream be given a chance. To this end, he founded and manages MBC BioLabs network, which now has four sites in San Francisco and San Carlos that are home to 117 companies. These co-working labs allow startups to be fast, focused, and frugal. Rather than spending months getting a facility up and running, MBC BioLabs startups can do experiments in their first week.
Doug is also a Managing General Partner of Mission BioCapital and has overseen the investment in 45 companies, many of which have already enjoyed successful exits (for instance, Alector, Atreca, Cell Design Labs, iPierian, Mitokinin, Principia, and Redwood Biosciences). He is a board member of Alessa, Avexegen, Epiodyne, Graphwear, Invenio, Myka, Magnap, SiteOne (observer), and Tangible Sciences.
Feng Han, MBA, Managing Partner, CTIC Capital
Feng Han has 30 years of healthcare operational and investment experience on a global basis in MedTech, pharma/biotech, and healthcare service sectors. His professional experiences include Partner at Pagoda Capital, an established global technology and healthcare investment firm headquartered in Beijing; Managing Director at Fosun Group based in New York, responsible for overseas healthcare private equity investment for one of the largest asset management firms from China; Director Corporate Business Development at Henry Schein, where he led M&A activities in Asia Pacific on behalf of one of the Fortune 500 MedTech companies in the US. He previously also had management positions with Sanofi Pasteur and R&D experiences in vaccine industry in China. Mr. Han currently is a member of the Board of Directors of Hamilton Thorne Ltd., a US public MedTech company.
Feng holds MBA in Finance and Marketing from The Wharton School, University of Pennsylvania, MS in Microbiology and Molecular Genetics from Michigan State University, and BS in Microbial Engineering from Shandong University.
Feng resides in Long Island New York and is an avid skier in the winter and an enthusiastic cyclist in the summer. He enjoys reading, travel, and going on long hikes with wife and three children.
Lu Yin, PhD, Founder and CEO, Persperion Diagnostics
Lu Yin received his Ph.D. in nanoengineering at the University of California San Diego, published>40 articles and patents on his research on non-invasive sensors and integrated wearable systems, and was selected as Forbes 30 under 30 in the Science category. In 2022, Lu founded Persperion Diagnostics Inc., aiming to use a novel non-invasive touch-based sweat biomarker sensing technology to help over 500 million diabetes users live happier and healthier lives, starting with replacing the painful and inconvenient fingerprick glucose sensors.
Michael L. Salgaller, PhD, Supervisor, Technology Analysis and Marketing Unit, NCI/NIH
Dr. Michael Salgaller leads the Technology Analysis and Marketing Unit (TAMU) within the National Institutes of Health (NIH’s) Technology Transfer Center, where he leverages over 20 years of business, scientific, and investment experience in various life science sectors to support technology development and commercialization. The TAMU serves in a business development role to foster licensing and collaborative activity between buy-side stakeholders and the NIH. Immediately prior to returning to the NCI, Michael was a Vice President at The Conafay Group, a healthcare-focused government affairs firm in downtown DC, where he led alliance development activities centered on civilian health. He served as President of Biologics Consulting Group, a boutique firm providing professional services focused on regulatory affairs. Michael spent several years on the investment team of an early-stage venture capital firm dedicated to the life sciences. He began his buy-side career as Vice President of R&D for Northwest Biotherapeutics, where he led the development of cancer cell therapies that achieved clinical benefit. He began his career as a Senior Scientist with Dr. Steve Rosenberg at the NCI. He is the author of “Biotechnology Entrepreneurship,” and teaches an entrepreneurship class at NIH. He is on the Board of Canines-N-Kids, a foundation supporting cooperation between researchers in pediatric and veterinary oncology. He has written over 70 scientific/business articles and book chapters. Dr. Salgaller received his PhD in Pathology from The Ohio State University.
Weian Zhao, PhD, Founder and CEO, Aureka Biotechnologies
Dr. Weian Zhao is the CEO of Aureka Biotechnologies and was previously a tenured full professor at UC Irvine. Throughout his career, Dr. Zhao has been pursuing disruptive innovations that have the potential to enhance human health. At Aureka, Dr. Zhao is leading a group of passionate entrepreneurs and innovators who dare to transform the unsustainable pharmaceutical industry by digitalizing and democratizing therapeutic discovery and development. Dr. Zhao co-authored approx. 100 articles in respected journals, including Science Translational Medicine, Nature Biomedical Engineering, Nature Communications, and PNAS. Dr. Zhao has received numerous awards, including MIT’s Technology Review TR35 Award, NIH Director’s New Innovator Award, World Economic Forum Young Scientist, and UCI Innovator of the Year. Dr. Zhao was trained as a bioengineer and pharmaceutical scientist at Harvard Medical School, Brigham and Women’s Hospital, MIT, McMaster University, and Shandong University.
Panel Discussion #2: Unveiling Life Sciences Transactions - Opportunities, Business Terms, and Practice Insights
Janet Xiao, PhD, JD, Partner, Morrison & Foerster LLP; President of CABS 2011-2012
Janet focuses her practice on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies. Janet’s clients range from large multinational biopharmaceutical companies, such as Celgene and Genentech, to emerging startup companies around the world. She advises her clients on patent matters relating to various technologies, including antibody therapeutics, cell therapeutics, nanomedicine, gene therapy, drug delivery systems, diagnostics, and nutraceuticals.
Janet works extensively in performing IP due diligence reviews in the contexts of VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. She helps some of the world’s most-sophisticated biopharmaceutical companies and investors assess potential risks and devise preemptive risk-management strategies for their product development and investments. She has led hundreds of IP due diligence projects, and is noted for her in-depth knowledge of patent landscapes in the fields of cancer immunotherapy, gene editing, next-generation sequencing, liquid biopsy, antibody manufacturing, and medical devices. Janet works regularly with patent litigators in the event of legal challenges by third-party competitors or vice versa, and she is frequently called upon to represent clients in litigation and post-grant proceedings.
Ario Arabi, MS, Executive Director of Global BD, Licensing Transaction, Novartis
Ario Arabi is a BD&L Transactions Executive Director at Novartis since June 2021. He has a wealth of transactional expertise with his most recently announced deal at Novartis being the collaboration and license with Ionis for discovery, development and commercialization of next generation program targeting Lp(a). Prior to his role at Novartis, he was Director of Corporate Development at Merck where he focused on oncology M&A.
Ario also brings 8 years of capital markets experience, as an investment banker at renowned firms such as Cowen and Stifel. Here, he supported numerous life sciences companies with financial advisory and capital raising services, including buy side/sell side processes as well as, equity and debt offerings. During the early part of his professional career, Ario worked in equity research in the biotechnology, pharmaceuticals, and medical devices sectors at various firms including Leerink and Citigroup. Ario holds a Bachelor of Science from Cornell University with a Double Major in Applied Economics & Management and Biological Sciences.
Julian Temple, MA, Managing Director, Lazard's Healthcare Group
Julian Temple is a Managing Director in the Healthcare Group in New York, having previously spent time in the firm's San Francisco and London offices. He works on a broad range of financial advisory assignments in the biotechnology and pharmaceutical sectors. Julian's transaction experience covers a wide range of strategic assignments including mergers and acquisitions, divestitures, alliances, joint ventures and take-private transactions. He has also been involved in capital raising, shareholder activism and financial restructuring. Julian has an M.A. in Natural Sciences from the University of Cambridge.
Madoo Varma, PhD, VP, Strategy& Business Development, DNA Script
Dr. Varma has straddled not only R&D, and business roles successfully but has had the unique experience of working in traditional biotech/diagnostics/life sciences tool companies as well as straddling across high tech companies focused on bioelectronics and digital health.
She earned her PhD in Genetics and went on to become a Common-Wealth Post Doctoral Scholar at Cambridge University, UK followed by 10+ years in Biotech R&D in Silicon Valley, California at GeneLabs, Adeza Dx, and Stanford University, working as senior scientist/director on target discovery, preeclampsia and pre-term labor prediction tests and infectious diseases, respectively.
She leveraged her rich technical background in genomics, proteomics and cell biology to transition to business roles. In the last two decades, she has held senior leadership roles in product management and business development at HP/Agilent, Applied Biosystems (Thermo), Intel, Labcyte (Danaher), and Codex DNA. AT HP/Agilent, she was instrumental in launch of microarray product line in addition to being part of the team that negotiated the Rosetta/Agilent alliance. At Applied Biosystems, she was a key member of the company strategy team and did several deals from sourcing to deal close. At Intel, as a GM of DNA sequencing incubation group, she led the team and cross institution alliance that led to $5M award for 1000$ genome sequencing. She led the early access collaboration agreement deal with Pfizer (for vaccines and Tx) that lead to a multimillion-dollar deal for Codex DNA.
At DNA Script, as VP of strategy and business development, she is focusing on partnerships with academia, pharma and tool companies to enhance and leverage company’s portfolio.
Matt Karlyn, JD, Partner, Morrison & Foerster LLP
Matthew Karlyn has 25 years of experience in working with companies in the healthcare, pharmaceutical, medical device, and technology industries on a wide range of commercial life sciences, licensing, and technology transactions. His clients range from Fortune 100 companies to start-ups, and he regularly advises companies on matters involving IP commercialization, complex collaboration transactions, licensing initiatives, subscription-based economics, and business transactions related to the procurement, development, commercialization, and use of technology and life sciences products. He has also worked with a number of clients on corporate transactions including mergers and acquisitions, as well as private equity and venture capital financing.
Life sciences clients, including pharmaceutical, medical device, and healthcare companies, rely on Matt for his breadth of knowledge in licensing, collaboration, and commercialization strategies across a wide range of transactions designed to strengthen operations and maximize value. Matt’s clients come from sectors across the global economy and include emerging companies, privately held companies, and many of the world’s biggest brands.
Matt has broad experience with commercial transactions and agreements in the life sciences space, including:
•Intellectual property and technology licenses
•Collaboration and development transactions
•Commercialization agreements
•Supply and manufacturing transactions
Shanshan Xu, PhD, VP, External Innovations, BioNTech
Shanshan Xu is head of Global External Innovations at BioNTech, where she and her team look for best-in-class assets and technologies to transform BNT’s pipeline. Her team works to bring on board those with the research skills necessary to help management develop our vision and give the company a sneak peek at where the industry is headed next.
External Innovations’ accomplishments include the partnership of Duality and OncoC4 - both Tier 1 oncology programs. The team is constantly on the lookout for innovative medicines and transformative science and believes that open minds are crucial to finding the best and most innovative partnerships for BioNTech.
Originally from Harbin, China, Shanshan spent almost half of her life in Mainland China, where she received her medical degree. She also holds a Ph.D. from UC-Irvine and an MBA from MIT. She spent a few years working on Wall Street before getting a call from Ugur Sahin to join BioNTech in 2020.
Slanix Paul Alex, Pharm. D, Portfolio Manager, Ally Bridge Group
Slanix Paul Alex joined Ally Bridge Group in 2023 and is Portfolio Manager for its public equity strategy. Before joining Ally Bridge Group, Slanix invested in life sciences companies as founding Partner and Senior Analyst for Tri Locum Partners and previously as an Investment Analyst for Consonance Capital Management. Prior to the buy-side, Slanix worked in sell-side biotechnology equity research at RBC Capital Markets and Credit Suisse. Slanix began his career in strategy consulting at Bionest Partners, advising life sciences companies on business development and commercial strategy. Slanix is a licensed pharmacist and holds a PharmD from St John’s University.
For your reference, here is some information about our previous Investor Forums:
We express our sincere gratitude for the generous sponsorship and support from Morrison & Foerster LLP.
We look forward to seeing you at the 2024 CABS Investor Forum.
Click HERE or scan the QR code below to register, please!

Kindly note that : There are limited seats at Morrison & Foerster. Each year many people are on the waiting list of CABS Investor Forum.
Registration and a photo ID are required to enter the venue (there will be a security check).
Morrison Foerster’s San Francisco office (425 Market St, San Francisco, CA 94105)
CABS is a volunteer-based, non-profit organization headquartered in San Francisco, committed to advancing the life sciences and biotech industry through education, collaboration, and networking.
The annual event, Investor Forum, held in conjunction with the JP Morgan Healthcare Conference, has been a hallmark of CABS for nearly 15 years. This year's forum is scheduled to take place on January 10th, 2024, from 8:00 am to 1:30 pm at Morrison Foerster’s San Francisco office (425 Market St, San Francisco, CA 94105). Due to venue capacity restrictions, the forum will have both on-site and online attendance, expecting over 130 on-site participants and more than 100 online attendees.
Our hybrid format will include two insightful panel discussions covering essential topics such as entrepreneurship, investment trends, licensing, and strategic considerations, as well as new opportunities and challenges in life sciences.
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105
The Chinese American Biopharmaceutical Society (CABS) kicked off 2024 with the Investor Forum on January 10 during the week of JP Morgan. The event was held in San Francisco at the offices of Morrison and Foerster. Morrison and Foerster, a CABS Sponsor, has partnered with CABS for over 10 years to host this event. This year’s themes focused on investments for 2024 and beyond as the industry emerges from a winter of investments from 2023 and identified strategies for transactions with opportunities, business terms, and lessons from 2023.

Jessica Sun, PhD, Executive Director, In Vivo Pharmacology, Terremoto Biosciences, President of CABS opened the event in a packed house with over 150 attendees.

Alex J. Zhang, PhD., CEO OneTwenty Therapeutics, CABS President 2017-2018; moderated the first panel which shared insights from Investors and Entrepreneurs as lessons learned from 2023 and provided guidance and strategies for funding and building a successful startup in 2024.
Perspectives from panelists highlighted the relationship building and branding for entrepreneurs, investors including the NIH program officer, strategic spending and buffering appropriately for the long haul to support development and manufacturing until clinical data read out, as well as diversity and inclusion in clinical trials.
2023 was a winter for funding and investing in pharmaceutical and biotechnology companies. It highlighted that there were less funds available for investment as it was driven by interest rates. Companies were propping up their current portfolio and prices were not adjusting to the valuation. Spending got out of control as capital was easy to come, but the burn rate was too high which made the funding in 2023 hard which resulted in layoffs. Prices for public companies dropped by 50%, but private companies did not change in equity and valuation. This was critical for 2023 in why funds were limited. Panelists encouraged entrepreneurs to focus on relationship building and be persistent. They advised not giving up and making the call as investors will invest. Wisdom was provided to be strategic in spending and allow the business to shrink space in need and have a sensitivity to capital spending. First time fundraising is hard and what you do not know you do not know; hence it is important to start talking to investors before you start raising money. Incubators can help. When the time comes to raise money then it becomes a more natural conversation. Establishing trust in the company and what the product is. Start early to build that connection. Do not think of asking for money from investors, but a connection as they can help on a conversation or connection in the future. Listen to your gut and read people to use them for the task at the company. This goes with the investors; they have a certain style or process. Work with an investor that you feel right with. Scientific and clinic boards will be people who will tell you what you need to hear and not what you want. You want to know the gaps and holes in an investment pitch. It was encouraged to diversify people in a company and have people who disagree and hold you accountable. Mentors, advisors, consultants, and board members can help in difficult situations as they can help de-risk.
Panelists encouraged entrepreneurs to focus on relationship building and be persistent. They advised not giving up and making the call as investors will invest. Wisdom was provided to be strategic in spending and allow the business to shrink space in need and have a sensitivity to capital spending. First time fundraising is hard and what you do not know you do not know; hence it is important to start talking to investors before you start raising money. Incubators can help. When the time comes to raise money then it becomes a more natural conversation. Establishing trust in the company and what the product is. Start early to build that connection. Do not think of asking for money from investors, but a connection as they can help on a conversation or connection in the future. Listen to your gut and read people to use them for the task at the company. This goes with the investors; they have a certain style or process. Work with an investor that you feel right with. Scientific and clinic boards will be people who will tell you what you need to hear and not what you want. You want to know the gaps and holes in an investment pitch. It was encouraged to diversify people in a company and have people who disagree and hold you accountable. Mentors, advisors, consultants, and board members can help in difficult situations as they can help de-risk. The discussion closed with predictions for 2024. Artificial Intelligence will take over sooner than we think. Consumers will be less patient as technology tools to make us know about ourselves increase and the need for instant feedback will grow as the speed and spread of information also increases because of technology. Deals announced the week of JP Morgan reflected big pharmaceuticals as they are being evaluated by stockholders return which will look more positive for 2024. Burn rate for a company will be the focus as things will take longer in this environment which included hiring, funding, and an FDA submission. Prepare yourself and reserve a buffer and cash. Pricing on drugs will also impact innovation. The hope is that post-election there will be bipartisan support for medical research. Antibody Drug Conjugates will not be the next hot topic. What is the next hot therapeutic side potentially neuroscience that can be driven with med tech may be ahead of Biopharma. A legation change can also bring back neglected diseases which could also change the industry to bring that back as a new push.
The discussion closed with predictions for 2024. Artificial Intelligence will take over sooner than we think. Consumers will be less patient as technology tools to make us know about ourselves increase and the need for instant feedback will grow as the speed and spread of information also increases because of technology. Deals announced the week of JP Morgan reflected big pharmaceuticals as they are being evaluated by stockholders return which will look more positive for 2024. Burn rate for a company will be the focus as things will take longer in this environment which included hiring, funding, and an FDA submission. Prepare yourself and reserve a buffer and cash. Pricing on drugs will also impact innovation. The hope is that post-election there will be bipartisan support for medical research. Antibody Drug Conjugates will not be the next hot topic. What is the next hot therapeutic side potentially neuroscience that can be driven with med tech may be ahead of Biopharma. A legation change can also bring back neglected diseases which could also change the industry to bring that back as a new push.
Panel Discussion 1: Reading the Tea Leaves for Biotech Investment in 2024 and Beyond (left to right)
Moderator: Alex J. Zhang, PhD., CEO OneTwenty Therapeutics, CABS President 2017-2018
Panelists: Douglas Crawford, PhD, Managing Partner, Mission BioCapital General Manager, MBC Biolabs; Feng Han, MBA, Managing Partner CTIC Capital; Michael L. Salgaller, PhD, Supervisor, Technology Analysis & Marketing Unit, NCI/NIH; Lu Yin, PhD, Founder and CEO Persperion Diagnostics; Weian Zhao, PhD, Founder and CEO Aureka Biotechnologies
CABS: Jessica Sun, PhD, Executive Director, In Vivo Pharmacology, Terremoto Biosciences, President of CABS 2024; Kay Tong, MA, Head of Quality Systems and Compliance, Sana Biotechnology, President-Elect 2024.
Janet Xiao, PhD, JD, Partner, Morrison & Foerster LLP, President of CABS 2011-2012 moderated the second panel discussion focusing on the fact that there had been more than 50 transactions representing over 150 million dollars including licenses and mergers by the panelists. This highlighted the fact that deals were happening in 2023. But was the driving factor and what were the strategies that were deployed for such success in 2023? Panelists shared that there are many large pharmaceutical companies that are potentially going to lose revenue in 2025-2030. To fill this gap, lackluster products, Mergers & Acquisitions (M&A) will come back and be strong to help fill the gap as pharma has been late and pressured to fill it. New modalities and new technologies will catch up as companies will try new things. Strategies include risk behavior of the big pharma on the earlier side of companies in development, which may include a 50/50 share whereas late-stage assets will get a better deal with an M&A. 2023 focused on money and the cost was high for new things and the process to get money was harder for the smaller companies. You had to give up some assets to get to the end goal to get funded in 2023. This also included different structures to access innovation and the flavor of the details with different incentives. It was also noted that there is always business development and the large pharmaceutical companies also have their own in-house research at which they can quickly help move an asset to commercial quickly. Hence, it is important to weigh out R&D study costs and to think about what needs to be put on the shelf to move other products through. This strategy explains why large pharmaceutical companies move to license on an earlier asset to de-risk their own portfolio and to keep more cash as an acquisition is more costly to enter industry.
Panelists shared that there are many large pharmaceutical companies that are potentially going to lose revenue in 2025-2030. To fill this gap, lackluster products, Mergers & Acquisitions (M&A) will come back and be strong to help fill the gap as pharma has been late and pressured to fill it. New modalities and new technologies will catch up as companies will try new things. Strategies include risk behavior of the big pharma on the earlier side of companies in development, which may include a 50/50 share whereas late-stage assets will get a better deal with an M&A. 2023 focused on money and the cost was high for new things and the process to get money was harder for the smaller companies. You had to give up some assets to get to the end goal to get funded in 2023. This also included different structures to access innovation and the flavor of the details with different incentives. It was also noted that there is always business development and the large pharmaceutical companies also have their own in-house research at which they can quickly help move an asset to commercial quickly. Hence, it is important to weigh out R&D study costs and to think about what needs to be put on the shelf to move other products through. This strategy explains why large pharmaceutical companies move to license on an earlier asset to de-risk their own portfolio and to keep more cash as an acquisition is more costly to enter industry. Another strategy discussed was diversification. Previously the market and trend focused on oncology and the shift is changing in 2024 to immunology and neurology. This also includes orphan indications as there are some big pharma that do not have that funding limitations to fill a gap hence, they can do longer investments. For example, Lily and Novo are looking ahead to other novel therapeutics. Aging was also discussed as a new push which included various aspects of aging such as stopping, slowing, delaying the effects; and treating age related diseases which include obesity and cardiovascular risk. The change is coming as leadership changes in these big pharmaceuticals as it will create a change for a new direction in a therapeutic avenue.
Another strategy discussed was diversification. Previously the market and trend focused on oncology and the shift is changing in 2024 to immunology and neurology. This also includes orphan indications as there are some big pharma that do not have that funding limitations to fill a gap hence, they can do longer investments. For example, Lily and Novo are looking ahead to other novel therapeutics. Aging was also discussed as a new push which included various aspects of aging such as stopping, slowing, delaying the effects; and treating age related diseases which include obesity and cardiovascular risk. The change is coming as leadership changes in these big pharmaceuticals as it will create a change for a new direction in a therapeutic avenue.
The climate is also changing in China as innovation is booming and scientists are going back to China as the problem of the U.S. and EU Bamboo ceiling has been experienced by many Chinese scientists. This has caused many to go back and start companies in China.  Exit strategies were also highlighted to advise entrepreneurs to be strategically planning early on as Chinese investors want to have the Initial Public Offering (IPO) on the Hong Kong or Shanghai exchanges. Whereas United States investors would want to exit on the NASDAQ. Hence, when deals or discussions begin it is recommended to seek outside counsel with appropriate expertise in the respective areas that also includes expertise in the local country for the transaction or asset as they are aware of the local economic trends.
Exit strategies were also highlighted to advise entrepreneurs to be strategically planning early on as Chinese investors want to have the Initial Public Offering (IPO) on the Hong Kong or Shanghai exchanges. Whereas United States investors would want to exit on the NASDAQ. Hence, when deals or discussions begin it is recommended to seek outside counsel with appropriate expertise in the respective areas that also includes expertise in the local country for the transaction or asset as they are aware of the local economic trends.
It was also highlighted to not drag out deals as the appetite may change for either side or due to trends or events happening around the world. Advice was also provided by the panelist to have reasonable transactions and understand the risk including having good due diligence activities and to trust your instincts. Panelists shared that the transaction is the beginning of a relationship, and it is important to build trust and credibility including sharing problems early on and listening to the needs of the other party. Preparation is the key to understanding the relationship as a collaboration or partnership. This includes a governance committee as well. Panel Discussion 2: Unveiling Life Sciences Transactions: Opportunities, Business Terms, and Practice Insights (left to right)
Panel Discussion 2: Unveiling Life Sciences Transactions: Opportunities, Business Terms, and Practice Insights (left to right)
CABS: Jessica Sun, PhD, Executive Director, In Vivo Pharmacology, Terremoto Biosciences, President of CABS 2024; Kay Tong, MA, Head of Quality Systems and Compliance, Sana Biotechnology, President-Elect 2024.
Moderator: Janet Xiao, PhD, JD, Partner, Morrison & Foerster LLP, President of CABS 2011-2012
Panelists: Ario Arabi, MS, Executive Director of Global BD Licensing Transaction, Novartis; Madoo Varma, PhD, VP Strategy & Business Development, DNA Script; Julian Temple, Managing Director, Healthcare Group, Lazard; Shanshan Xu, PhD, VP, External Innovations, BioNTech; Matt Karlyn, JD, Partner Morrison & Foerster LLP; Slanix P. Alex, PharmD, Portfolio Manager, Ally Bridge Group
CABS strategically had over hours of networking opportunities for both entrepreneurs and investors to enable collaboration opportunities. Lunch closed the event which was an hour of networking as well.

 Special thanks to our CABS Volunteers as the event could not have been made possible without their help. CABS also acknowledge the successful promotion and attendance made possible for this event due to the dedication and commitment of our CABS PRC.
Special thanks to our CABS Volunteers as the event could not have been made possible without their help. CABS also acknowledge the successful promotion and attendance made possible for this event due to the dedication and commitment of our CABS PRC.
CABS Volunteers:
Jessica Sun, PhD,President 2024; Kay Tong, MA, President-Elect 2024; Yuying “Kate” You, PhD, JD, Past President 2023; Li Wang, MS, SLC Co-Chair; Liping Meng, PhD, BCD Co-Chair; Liang He, PhD, PRC Co-Chair; Lin Wang, PhD, Volunteer; Lu Lu, MS, AMC Co-Chair; Shicheng Guo, PhD, BCD EC; Shirley Dou, MS, Volunteer; Yan Wang, PhD, STC Co-Chair; Zhiqing Wang, PhD, Volunteer


Acknowledgements:
Recap Author: Kay Tong, MA, CABS President-Elect 2024
Editors: Liping Meng, PhD, BCD Co-Chair; Jessica Sun, PhD,CABS President 2024
Photographers: Lu Lu, MS, AMC Co-Chair; Li Wang, PhD, SLC Co-Chair
Event Promotion: Liang He, PhD, PRC Co-Chair
Morrison and Foerster, SF office
CABS is pleased to announce Kay Tong as CABS 2024 President-elect!

Kay is currently the Head of Quality System & Compliance at Sana Biotechnology, Inc. She has over 20 years of management experience in pharmaceutical and medical devices companies including areas of research, manufacturing, quality control, and quality assurance. Before the current position, she had served multiple leadership roles in Apollomics, Gilead Sciences and Life Technologies. In additional to her successful career, Kay also has dedicated countless volunteer hours to professional and community organizations. For example, she is passionate about mentoring women to guide their career while balancing life and breaking cultural norms.
Kay joined the CABS as an active member in 2019, and quickly started to serve as a key member at executive council. She has served as the Co-Chair of the Business and Career Development (BCD) Committee since 2020. During these years, she has hosted several career development series which have been very well received by the CABS community. She is also well recognized as an inspirational speaker and mentor at CABS’ flagship program, Career Advisory Network (CAN) program. She has received multiple awards from CABS, such as Outstanding Service Award for 2020, Co-Chair Contribution Award for 2022. Kay has a vision of growing CABS members passions for serving and leadership to grow the next generation of the leaders in the community.
Please join us to welcome Kay Tong as CABS 2024 President-elect!
With Kay being elected as 2024 President-elect, Dr. Jessica Sun now becomes the 2024 President of the CABS. Let’s congratulate them both!
CABS celebrated the Career Advisory Network (CAN) Class 2023.
On November 11, 2023, members of the CABS EC, along with mentors and mentees, gathered to celebrate the graduation of CAN Class 2023. The program attracted about thirty mentors and over fifty mentees this year. Kay Tong, Chair of the Business & Career Development Committee, took charge of reviewing and moderating the event. During the session, mentors and mentees alike shared their enriching six-month journey, delving into both the benefits and challenges encountered.
Mentors highlighted that the experience was not just an opportunity for them to contribute to the next generation but also a chance for continuous learning from their mentees. The presentations revealed a wealth of key takeaways and lessons. Mentees, spanning various career stages, expressed their gratitude for being paired with mentors who guided them in strategic navigation, effective communication, and overall career growth. Many were thrilled at the prospect of the program continuing into 2024, with some mentees even expressing interest in transitioning to become mentors themselves.
In a gesture of appreciation, mentors received thoughtful gifts, while mentees were presented with certificates marking their successful completion of the program. CABS provided lunch, fostering an environment where mentors and mentees could continue to grow within the realm of career networking.
CAN, situated within the Career and Business Development group in CABS, operates as a one-to-one mentoring program. It aims to guide mentees across various career paths, including business development, traditional scientific roles in academia and industry, and entrepreneurship. As a free program for CABS members, the success of the mentorship initiative is attributed to the hard work, dedication, persistence, and service of mentors who, amidst their busy schedules, carve out time to guide and uplift mentees.
Hanhai-1633 Old Bayshore Hwy #280, Burlingame, CA 94010
CABS celebrated the Career Advisory Network (CAN) Class 2023
On November 11, 2023, members of the CABS EC, along with mentors and mentees, gathered to celebrate the graduation of CAN Class 2023. The program attracted about thirty mentors and over fifty mentees this year. Kay Tong, Chair of the Business & Career Development Committee, took charge of reviewing and moderating the event. During the session, mentors and mentees alike shared their enriching six-month journey, delving into both the benefits and challenges encountered.
Mentors highlighted that the experience was not just an opportunity for them to contribute to the next generation but also a chance for continuous learning from their mentees. The presentations revealed a wealth of key takeaways and lessons. Mentees, spanning various career stages, expressed their gratitude for being paired with mentors who guided them in strategic navigation, effective communication, and overall career growth. Many were thrilled at the prospect of the program continuing into 2024, with some mentees even expressing interest in transitioning to become mentors themselves.
In a gesture of appreciation, mentors received thoughtful gifts, while mentees were presented with certificates marking their successful completion of the program. CABS provided lunch, fostering an environment where mentors and mentees could continue to grow within the realm of career networking.
CAN, situated within the Career and Business Development group in CABS, operates as a one-to-one mentoring program. It aims to guide mentees across various career paths, including business development, traditional scientific roles in academia and industry, and entrepreneurship. As a free program for CABS members, the success of the mentorship initiative is attributed to the hard work, dedication, persistence, and service of mentors who, amidst their busy schedules, carve out time to guide and uplift mentees.
Hanhai:1633 Old Bayshore Hwy #280, Burlingame, CA 94010
Chinese American Biopharmaceutical Society (CABS) Honors Dr. Corey Goodman with the 2023 K. Fong Award in Life Sciences
South San Francisco, CA - The Chinese American Biopharmaceutical Society (CABS) proudly announces Dr. Corey Goodman as the recipient of the prestigious 2023 CABS K. Fong Award in Life Sciences, commemorating the 10th anniversary of this honored distinction. This esteemed award recognizes Dr. Goodman's outstanding contributions to science, education, entrepreneurship, and venture capital. The award will be presented during the 2023 BioPacific Conference on October 28th, 2023, at the South San Francisco Convention Center.
Dr. Corey Goodman
A celebrated figure in the scientific community, Dr. Corey Goodman's journey has been nothing short of remarkable. He obtained his B.S. from Stanford University and earned his Ph.D. from U.C. Berkeley. He spent 25 years as a Professor of Biology at Stanford and a Professor of Neurobiology at Berkeley where he was a Howard Hughes Medical Institute Investigator, co-founder of the Wills Neuroscience Institute, and achieved an h-index of 131. Dr. Goodman is currently the Adjunct Professor of Neuroscience at Berkeley.
He is an elected member of the National Academy of Sciences, American Academy of Arts and Sciences, and American Philosophical Society, and a recipient of many honors, including the Alan T. Waterman Award, Canada Gairdner Biomedical Award, March-of-Dimes Prize, and Gruber Prize in Neuroscience.
Dr. Goodman's achievements extend beyond academia. He transitioned from research to the biotechnology industry, bridging the gap between scientific discoveries and impactful medicines. He co-founded eight biotech companies, notably Exelixis being the first one; he served as the CEO of Renovis, leading the company to go public and achieve a successful acquisition by Evotec. At Pfizer, he was President of the Biotherapeutics and Bioinnovation Center and a member of the executive leadership team.
As Managing Partner of venBio, a venture capital firm he co-founded, Dr. Goodman fosters groundbreaking scientific advancements. He chairs the Boards of leading biotechnology companies like ALX Oncology, Tallac, Axonis, Attralus, MindRhythm, Axent, and Insamo. Additionally, he is a valued member of the Boards of NFlection and FogPharma. He chaired Labrys Biologics, a company he founded, until its acquisition by Teva. The Labrys drug, called Ajovy, is a CGRP antibody for chronic migraine that was approved by the FDA in 2018.
His commitment to public service is commendable. As the former Chair of the California Council on Science and Technology, Dr. Goodman provided invaluable guidance to the Governor and State Legislature. He also served as the former Chair of the National Research Council's (NAS) Board on Life Sciences, advising the Federal Government on critical matters.
CABS honors Dr. Corey Goodman's exceptional accomplishments and profound impact on the life sciences industry and academia. His visionary leadership, groundbreaking research, and dedication to healthcare have left an indelible mark on the scientific community.
For media inquiries and press registration for the award ceremony, please contact: info@cabsweb.org
About CABS K. Fong Award in Life Sciences
The CABS K. Fong Award in Life Sciences is an esteemed annual recognition presented to individuals who have made exceptional contributions to the fields of life sciences and the biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates eligible for this award must be nominated by an active member of CABS. Selection criteria are based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
-
Proven achievements in therapeutic breakthroughs (including discovery, process, or clinical development), diagnostics or research reagent/equipment markets.
-
Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
-
Significant contribution to the CABS community and promotion of international collaborations in life sciences.
The CABS K. Fong Award in Life Sciences aims to celebrate and honor exceptional individuals whose achievements and commitment have not only expanded the frontiers of life sciences but have also positively impacted global healthcare and well-being.
About Dr. Kenneth Fong
Dr. Kenneth Fong has spent the last 39 years in the biotech industry after completing his academic pursuit in biomedical research.
He is best known for founding the biotech company, Clontech in 1984 which he built into one of the largest biomedical tool companies founded by an Asian American in the US (400 employees including 65 PhD scientists). Clontech was acquired by Becton Dickinson in 1999 and Ken has continued his career as a Venture capitalist with Kenson Ventures that he founded. He has since cultivated 12 highly successful entrepreneurs, advising them and working with them on the growth of their companies.
Currently, he sits on the board of 4 biotech companies and he was intimately involved with the M/A and IPO of more than 15 companies that are worth more than $7 billion. These companies range from research tools, medical diagnostics and drug development. In almost all cases, Dr. Fong has been instrumental in providing strategies for sustainable growth, value creation and liquidity. Those successful entrepreneurs have moved on to assume leadership in other start-up and mid-sized companies, which in turn led to a new generation of entrepreneurs.
Ken has held a number of leadership positions over the years. He served as the President of the Society of Chinese Bioscientists in North America (2006-2007) and President of the Bay Area Asian American Manufacturers' Association (AAMA, 1987). He was also a member of the Board of Trustees of the California State University System (2006-2013). His philanthropic interests include scholarships to San Francisco State University, the Kenneth Fong-Hearst endowed scholarships to the CSU system and 40 student scholarships to Peking University. In 2003, he was involved with establishing the Fong Optometry and Medical library at UC Berkeley, and more recently an endowed professorship at Stanford University and a technology translation endowed fund at San Francisco State University.
Ken obtained his PhD from Indiana University and his post-doctoral studies at UCLA & NIH.
Past recipients of CABS K. Fong Awards
2022: Dr. Scott Liu, Founder and CEO of HanchorBio, for his outstanding and pioneering contribution to the development of multiple biologic products from research to launch.
2021: Dr. John O. Link, Vice President of Gilead Sciences for his work on the drug discovery of Hepatitis C inhibitors and the HIV Capsid Inhibitor; and Dr. Xian-Ping Lu, Chairman, CEO of Shenzhen Chipscreen Biosciences Co. LTD, for their extraordinary achievements in research, innovation and entrepreneurship.
2019: John V. Oyler, Chairman, Co-Found and CEO of BeiGene, for his entrepreneurship and business leadership to establish BeiGene as a world-class biopharmaceutical company.
2018: Dr. Yuling Luo, Founder, CEO and Chairman of Alamar Biosciences (formerly the founder & CEO of Advanced Cell Diagnostics, ACD), and Dr. Guoliang Yu, Executive Chairman of Crown Bioscience, for their successful serial entrepreneurship in the life science business.
2017: Dr.Yinxiang Wang, Co-founder and CSO of Beta Pharma, for his role in leading development and commercialization of Conmana®, the first small molecule oncology drug specifically targeting cancer cells that was completely developed in China; and Dr. Edgar Engleman, for his pioneering research that was the basis of the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA.
2016: Dr. Gerald Chan, Co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, Professor of Stanford University, for his pioneering work in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for pioneering and shaping the CRO business model in China; and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life-saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth of Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market; and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.
South San Francisco Convention Center
We are honored to host the 2023 BioPacific Conference(aka the CABS 24th Annual Conference) on October 28th, 2023 (Saturday).
The theme of the conference is "Collaboration, Acceleration, Breakthrough, & Success - 25 Years of CABS".
Over 600 attendees are expected globally, from the life science and Biopharmaceutical industries. We are also offering sponsorship opportunities, please contact fundraising@cabsweb.org for more information.

please contact fundraising@cabsweb.org or visit https://www.cabsweb.org/sponsors/ (or scan the QR code below)for more information.

South San Francisco Convention Center
Please Click Here to Register!
.png)
CABS 2023 BioPacific Conference
Collaboration, Acceleration, Breakthrough, & Success
- 25 Years of CABS
Click here to Downoad the 2023_BioPacific_Program Book .pdf
CABS is pleased to host the 2023 BioPacific Conference(aka the CABS 24th Annual Conference).
Date/Time: 8:00AM - 6:00PM PT on Saturday, Oct 28, 2023.
Location: South San Francisco Convention Center, 255 S Airport Blvd, South San Francisco, CA 94080
Limited sponsorship opportunities available. Please contact fundraising@cabsweb.org.
This year marks the 25th anniversary of Chinese American Bio/Pharmaceutical Society (CABS). As we reflect on our accomplishments, the sky of the biopharmaceutical industry is clouded with some pressing questions. What is the trend of drug discovery when the low-hanging fruits of single targets have already been picked? As ChatGPT has become an instantaneous household name, how is the industry going to harness the soaring power of generative AI to boost its growth? How and where are the investors going to invest in a season of capital scarcity? What are the best pieces of advice for career development in a time with no shortage of turbulence? The 2023 BioPacific Conference, with its stellar assembly of speakers/panels, would like to have these critical questions addressed. Here is a list of highlights of the 2023 BioPacific Conference:
-
Celebration of 25 years of remarkable community services of CABS in the birthplace of biotech.
-
Winner of the 2023 CABS K. Fong Award in Life Sciences: Dr. Corey Goodman
-
Keynote address: "Multispecific Medicines for The Future" by Dr. Raymond Deshaies, who pioneered PROTAC.
-
Keynote address: “De Novo Protein Design of Functional Proteins” by Dr. William DeGrado, who coined the term “de novo protein design” himself.
-
Cutting-edge technologies, such as gene editing, oral peptide, and innovative platform of cell therapy, in drug discovery and development, discussed by leading experts.
-
Investment strategies in biopharmaceutical industry in a challenging and uncertain time.
-
Role of artificial intelligence in drug discovery and development.
-
Career advice from past CABS presidents, all leaders and veterans of the industry.
-
Uncovering potential solutions and opportunities by networking with tens of speakers/panelists, business partners, and hundreds of attendees.
Please Click Here to Register!
Podium Speakers


Please Click Here to Register!
Agenda





Registration Fees
Registration online as early as possible is highly recommended.
The registration on October 28, 2023 and on-site will be $150.
|
CABS Member |
Early Bird Registration (by 10/11/2023) |
Regular Registration (10/12/2022- 10/26/2023) |
| General | $60 | $100 |
| Academic | $40 Please use your institution email (.edu) |
$80 Please use your institution email (.edu) |
| Non-Member* |
Early Bird Registration (by 10/11/12023) |
Regular Registration (10/12/2023- 10/26/2023) |
| General | $90 | $130 |
| Academic | $70 Please use your institution email (.edu) |
$110 Please use your institution email (.edu) |
* Refreshments, lunch are included.
* Join CABS today ($30 for one year membership) to save on registration fee: http://www.cabsweb.org/members/
* Please contact info@cabsweb.org, if there are any questions about registration.
Please Click Here to Register!
Special Shout-out to Our BioPacific Conference 2023 Sponsors
.png)
Thinking about becoming a sponsor of the BioPacific Conference? Please contact fundraising@cabsweb.org . Thank you very much!
2023 CABS BioPacific Conference sponsorship package

Please Click Here to Register!
South San Francisco Convention Center, 255 S Airport Blvd, South San Francisco, CA 94080

The 2023 Biopacific Conference, alternatively recognized as the 24th Annual Conference of the Chinese American Bio/Pharmaceutical Society (CABS), took place on October 28th, 2023, at the South San Francisco Conference Center in California. This exceptional gathering, commemorating the 25th anniversary of CABS, featured a notable lineup of speakers, panelists, and moderators, enjoyed generous support from 58 sponsors, and drew the participation of over 700 attendees. The remarkable success of this event owes its existence to the invaluable contributions and active engagement of our esteemed speakers, sponsors, attendees, members of the organizing committee, and dedicated volunteers.

The theme of the Conference was ‘Collaboration, Acceleration, Breakthrough, & Success - 25 Years of CABS’. The conference comprised 2 keynote addresses, a speech for the K. Fong Award, 5 scientific presentations, 3 dynamic panel discussions, and two interactive breakout workshop sessions, all led by prominent figures from both the industry and academia.

The conference commenced with an opening speech by Jessica Sun, MD, PhD, President-Elect of CABS and Chair of the Organizing Committee, who offered a warm welcome and an overview of the conference agenda. Kate You, JD, PhD, President of CABS, delivered the State of the Society address, spotlighting the society's activities, growth, and achievements over the past year.
Morning Session 1 – Session Chair: Yanan Wang, PhD, CABS Executive Council

This session featured three insightful talks. Dr. Ray Deshaies, Senior Vice President of Global Research at Amgen, a member of National Academy of Sciences and American Academy of Arts and Sciences, delivered a keynote presentation on the emergence of the fourth wave of Transformative Innovation, which follows the advent of molecular drugs, rational drug design, and the biotechnology revolution. He provided an insightful analysis of the challenges and opportunities in developing the next generation of multispecific small molecule and biologic medicines, and shed light on Amgen's pioneering approaches to multispecific medicine. His presentation illuminated the exciting frontiers of medical innovation, inspiring the audience about the future of medicine. Dr. Bianxiao Cui, Professor at Stanford University, presented the latest data showing that membrane curvature promotes the formation of a new type of integrin-mediated cell adhesions, termed as ‘curved adhesions’. These findings unveiled the fascinating mechanism of cell anchorage to soft protein fibers, offering insights into potential therapeutic targets for fibrosis diseases. Finally, Dr. Wei Huang, President of Henlius and Chairman of Aton Biotech, provided a comprehensive overview of the drug development process, covering CMC activities and key considerations from clinical to commercial stages.
Morning Session 2 – Session Chair: Lu Lu, MS, CABS Executive Council

Dr. Kenneth Fong presented the 2023 CABS K. Fong Award to Dr. Corey Goodman, Managing Partner at venBio and Adjunct Professor at UC Berkeley.

Dr. Corey Goodman, a member of National Academy of Sciences and American Academy of Arts and Sciences, emphasized the significance of a transformative ecosystem, which is comprised of pharmaceutical companies, biotech firms, academic institutions, strategic capital, and capital-efficient models, including incubators. Dr. Goodman underlined the strategic advantage gained by pharmaceutical companies when they leverage early discoveries and innovations from academia and biotech, resulting in substantial reductions in internal research and development expenditure. This strategy often involves the acquisition or licensing of proof-of- concept projects, followed by progression into late-stage trials, manufacturing, and commercialization.

The panel discussion, titled 'Ask the Investor: Glimpsing the Future of Life Science Innovation' and moderated by Dr. Ella Li, brought together a distinguished group of panelists, including Dr. Ronjon Nag, Dr. Ted Hou, Mr. Mike Ryan, and Mr. Tao Fu. They offered a comprehensive exploration of the dynamic landscape of the life science sector. The discussion illuminated a promising trajectory that hinges on investment, innovation, and collaborative efforts to propel the next wave of transformative breakthroughs in healthcare and life sciences.

Dr. Phillip Patten, CSO at Initial Therapeutics, gave a talk that provided insights into the remarkable journey of DiCE Therapeutics, from its inception in 2013 to its acquisition by Eli Lilly in 2023. Dr. Patten's presentation left the audience with key takeaways, emphasizing the importance of tackling difficult yet valuable problems with novel approaches and embracing high technical risk while maintaining a practical and strategic outlook when selecting target objectives.
Concurrent L & L Session I, Chair: Denna Kwang, CABS Executive Council

During the first ‘Lunch & Learn’ session, Dr. Liyu Wu, Senior Director of Business Development of Pharmaron, emphasized the company’s extensive manufacturing capabilities, designed to meet the diverse needs of clients at every stage of R&D. Following Dr. Wu's presentation, Bill Glencross, Director of Sales of Mispro, outlined their all-encompassing vivarium services and facilities, covering everything from animal husbandry to assisting clients with IACUC protocols. The session concluded with Yifu Liu, Executive Director of JJlake, who provided a historical overview of JJlake and spotlighted their cutting-edge facilities and innovative business model, which includes venture capital initiatives and potential partnerships.
Concurrent L & L Session II, Chair: Katherine Guan, CABS Executive Council

In the second ‘Lunch & Learn’ session, Dr. Karen Han, Field-Based Account Manager at GenScript, elaborated the extensive array of life science research services and products, highlighting their upgraded US facility for gene synthesis and their newly launched Singapore facility to support both gene synthesis and protein production. Next, Dr. Jiyong Zhang, Head of Business Development at Nona Biosciences, introduced Nona's proprietary Harbour Mice transgenic platform. He emphasized its integrated capabilities, particularly in the discovery and production of fully humanized heavy chain-only antibodies. Concluding this session, Dr. Yujiao Zhang, Product Marketing Scientist at Kactus, shared insights into the company's remarkable growth over the last five years. She highlighted Kactus' pivotal role in providing research-grade and GMP-grade products that drive innovation in the fields of biopharma and cell & gene therapy.
Afternoon Session 1 – Session Chair: Shicheng Guo, PhD, CABS Executive Council

This session consisted of two talks and one panel discussion. Dr. William DeGrado, a member of National Academy of Sciences and American Academy of Arts and Sciences, and a professor at UCSF, presented a keynote speech on the design approach for proteins binding to small molecules and their potential biomedical applications, such as drug delivery and drug reversals. To address another challenging aspect of de novo protein design - membrane proteins, Dr. DeGrado showcased the design of semi-synthetic membrane proteins acting as ion channels and their applications in DNA and RNA sequencing. Dr. Dinesh Patel, CEO of Protagonist Therapeutics, discussed the development of oral IL-23 receptor antagonists at Protagonist. They discovered of PTG-200, an oral gut-restricted IL-23 receptor antagonist peptide, led to a partnership with Janssen. Protagonist's platform has expanded treatment options for inflammatory or autoimmune diseases with the first oral IL-23 receptor antagonist peptide drug, marking a significant innovation in the field.

The panel discussion, ‘Leveraging Artificial Intelligence in Drug Discovery and Development: Opportunities and Challenges’, was moderated by Dr. Cheni Kwok, joined by Dr. Nicolas Tilmans, Dr. Stephanie Sharron, and Dr. Pek Lum. The panelists underscored the profound potential of AI to revolutionize and expedite drug discovery. The panelists also discussed numerous opportunities offered by this emerging frontier, including sifting through vast datasets to unearth novel drug targets, predicting drug-target interactions, constructing molecular structures with specific properties, emulating intricate biological systems, and refining the design of clinical trials.
Afternoon Session 2 – Session Chair: Liang He, PhD, CABS Executive Council

This CABS special session, which marks the celebration of CABS' 25th anniversary, commenced with a presentation by Dr. Cheng Liu, the Founder, President, and CEO of Eureka Therapeutics, and former President of CABS (2004-2005). Dr. Liu shared insights on the topic of "Developing Cell Therapy with the Capacity to Cure Solid Tumors." Dr. Liu's presentation demonstrated the superior safety and efficacy of ARTEMIS T cells, representing a significant advancement in the field of cell-based therapy for solid tumors.

The CABS special panel discussion, titled "To Stay Or To Go – Navigating Career Growth in an Ever-Changing Environment," featured a distinguished group of six former CABS presidents, including Dr. Jen Majeti (moderator, 2015-2016), Dr. Jennifer Hu (2006-2007), Dr. Shichang Miao (2009-2010), Dr. Janet Xiao (2011- 2012), Dr. Zhonghua Pei (2013-2014), and Dr. Alex Zhang (2017-2018). The discussion covered a range of career-related topics, such as the influence of their CABS experiences on their career trajectories, strategies for building and maintaining professional networks, the challenges of navigating careers in both big pharma and biotech startups, etc. The panel not only provided valuable insights and creative perspectives for young professionals but also resonated with the overarching theme of the 25th anniversary celebration. The session concluded with a memorable group photo featuring over ten past presidents, adding a touch of nostalgia and camaraderie to the event.

The conference concluded with a closing address by Dr. Keting Chu, the Founding President of CABS, followed by a fun iLOVE CABS Draw. In her closing remarks, Dr. Chu introduced the history of CABS, tracing its evolution from its inception to its current state and elucidating the mission that continues to drive CABS forward. She highlighted the pivotal role of collective dedication, expertise, and unwavering passion, emphasizing their instrumental role in advancing the biopharmaceutical industry. Dr. Chu also encouraged the audience to look ahead with optimism, as the future holds promising prospects for collaboration and innovation.

In closing, we wish to extend our heartfelt gratitude once more to all the distinguished speakers, enthusiastic sponsors and attendees, the hardworking organizing committee, and the dedicated volunteers who contributed to the resounding success of this conference. It was your unwavering commitment and boundless passion for CABS and the broader biopharmaceutical community that truly propelled this event to greatness. We are deeply appreciative of your collective efforts and look forward to the prospect of reconnecting with you in future events. Together, we will continue to drive collaboration, acceleration, breakthrough, and success within the field, shaping the future of the biopharmaceutical field.
South San Francisco Conference Center
The "Accelerating Drug Discovery and Disease Treatment Through Innovative Gene Editing Technologies" webinar, hosted by CABS on October 13 via a Zoom meeting, proved to be a remarkably informative and engaging event. The webinar assembled leading experts in the field to discuss cutting-edge advancements in gene editing, animal models, and gene therapy. Dr. Yuying (Kate) You, President of CABS, opened the webinar with welcoming remarks, setting the stage for an insightful and collaborative event.
Dr. Le Cong delivered an enthralling presentation titled "Beyond CRISPR: Metagenomic Mining of SSAP for In Vivo Precision Knock-In of Long Sequences." Dr. Cong's talk pushed the boundaries of gene editing, moving beyond CRISPR technology to unveil the potential of SSAP (sequence-specific endonucleases) for precision knock-ins of extensive DNA sequences within living organisms.
Dr. Shengjiang (Shawn) Liu's presentation, "AAV Capsid Engineering for Ocular Gene Therapy," shed light on the remarkable progress in adeno-associated virus (AAV) capsid engineering, particularly in the context of ocular gene therapy. His work highlighted the promise of novel treatments for ocular diseases.
Dr. Guangming Wu presented "Rapid Generation of ACE2 Humanized Inbred Mouse Model for COVID-19 with Tetraploid Complementation," demonstrating the swift development of an ACE2 humanized inbred mouse model critical to COVID-19 research. Dr. Wu emphasized the significance of tetraploid complementation in this process.
During a session on ongoing developments in gene therapy for β-Thalassemia, Dr. Lei Shi discussed "Gene Therapy for β-Thalassemia."
Dr. Jiaoyang Sun delivered an engaging talk on "The Application of Turbomice™ Technology in Animal Models." Dr. Sun explored the innovative Turbomice™ technology and its applications in creating advanced animal models for research, offering fresh perspectives in the field.
The webinar successfully attracted over 100 attendees, reflecting the widespread interest and importance of the topics discussed. With a diverse array of presentations, the event provided a comprehensive overview of the cutting-edge developments in gene editing and its applications in disease treatment and drug discovery.
Online Zoom meeting

Don't miss out on this sizzling summer event! Grab your family and friends, and let's BBQ together!
Thanks to our sponsors:
city boothbay park; Boothbay Ave &, Edgewater Blvd, Foster City, CA 94404

We would like to express our gratitude to the more than two hundred guests who attended, the past presidents of CABS, including the founding president, for their on-site support, and the sponsorship from Biocytogen, Sino Biologics, and SALA Homes & Associates. We also want to thank the two co-chairs from the Social Life Committee, and, of course, our most lovely forty volunteers.

Thanks a zillion to our sponsors
city boothbay park; Boothbay Ave &, Edgewater Blvd, Foster City, CA 94404
We regret to inform you that we have had to make the difficult decision to cancel the upcoming job fair that was scheduled to be held on 06102023. We apologize for any inconvenience or disappointment this may cause you, and we sincerely regret not being able to proceed as planned.
Thank you for being a valued member of our community.

Are you looking for new talents to join your team? Would you like to meet your potential employees face to face?
CABS Business and Career Development Committee is offering your company a FREE opportunity to recruit new talents. The in-person job fair will bring job seekers and employers together.
To participate, please fill in this form or scan the QR code below.
Please also post your company’s current open positions on http://www.cabs-system.com/ by May 20.

For sponsorship opportunity or participation for this in-person event please email to fundraising@cabsweb.org
Q-Bay Center at 160 E Tasman Dr., San Jose

Since being established in 2010, the Career Advisory Network (CAN) Program has become one of the major annual events of CABS. CAN is a mentoring program hosted by the Business and Career Development (BCD) committee of CABS. It is a direct one-to-one pairing mentorship program to guide mentees in their career paths including business development, entrepreneurship, and scientific career paths in both academia and industry. The kickoff meeting of the 2023 CABS CAN program took place on April 22, 2023, at Hanhai Silicon Valley Center. Fourteen mentors and 31 mentees attended the event.


Danielle Liu, advisor of the CAN program, emceed the kickoff meeting.

At the beginning of the meeting, Dr. Yuying (Kate) You, the 2023 CABS president, gave a brief introduction of CABS.

Subsequently Kay Tong, co-chair of BCD outlined the logistics of the program, the expectations for mentors and mentees. Kay also shared her thoughts on the importance of mentorship and how the CAN program can make a positive impact on both mentors and mentees. Her insights inspired attendees and set the stage for the rest of the meeting.

Dr. Shichang Miao, a successful entrepreneur and mentor, shared his career story and mentoring experience, and provided invaluable advices, such as whether and when to join a big or a small company.

Drs. Yuwei Yang and Hesong Sun, two former mentees, also shared their experiences and offered valuable advices, such as being open-minded, asking for clarification and providing timely feedback to mentors.


After the sharing session, mentors and mentees mingled together, strengthened old connections and established new ones.



Overall, the kickoff meeting was an excellent start for this exciting CAN program, and attendees left feeling empowered and motivated to achieve success. Special thanks to Jack Zhu and Dr. Xiaojie Chen, the coordinators of 2023 CAN program.
For any questions, please contact the chairs of the CAN Program Xiaojie Chen (xchenselleck@gmail.com) or Jack Zhu (jackningzhu@gmail.com)
Finally, we sincerely thank the exclusive sponsorship from Porton Advanced Solution, a cell and gene therapy CDMO company.
Porton Advanced Solutions is a leading end-to-end gene and cell therapy CDMO, committed to accelerating the development and manufacturing of advanced therapies to deliver life-changing medicines to patients. With over 220,000sqft of GMP facilities, we offer end-to-end services that cover process development, analytical development, and cGMP manufacturing of viral vectors, plasmid, mRNA, and cell products.
1633 Old Bayshore Hwy STE 280, Burlingame, CA 94010
Career Building Series
Strategically Mapping Your Career
March 25, 2023 2-4 pm
1633 Old Bayshore Hwy STE 280, Burlingame, CA 94010
FREE WORKSHOP
● I didn’t get that promotion, should I change my job?
● Not sure if I should change my career path?
● Why am I not going anywhere with my career?
Have these questions crossed your mind?
This workshop will provide you the tool to map out your career strategically.
1) Learn to identify your career goals
2) Map career timelines with lifestyle milestones
3) Understand where to pivot strategically to advance your growth
This is a live in person workshop. You will walk away with tools that you can utilize for your career growth. There will be an opportunity for you to build your own road mapSpeaker: Kay Tong, Head of Quality System and Compliance, Sana Biotechnology, Inc.
Panel: Sarah Kim Chen, Clinical Research Medical Director, Amgen
Panel: Yue Wang, Business Development Manager, Mission Bio
Registration:
https://www.cabsweb.org/registration/5205099/Strategically%20Mapping%20Your%20Career/
1633 Old Bayshore Hwy STE 280, Burlingame, CA 94010
Dear everyone,
Greetings from the Chinese American Biopharmaceutical Society (CABS), Business and Career Development committee!
Building upon the success of the CAN (Career Advisory Network) program last year, we have decided to launch the program again this year. We would like to extend a sincere invitation to you to join us as either a mentor or a mentee. The goal of CAN is to foster the development of future life sciences professionals and leaders through mentoring, skill enhancement, and networking opportunities. The program runs for a period of six months and offers one-on-one mentoring sessions, as well as small and large group networking events. Mentors and mentees are expected to meet once a month during the six months, either in-person or virtually.
Paring of mentors and mentees will be based on a variety of factors such as background, mutual interest, career goals, locations, etc. Program is scheduled to be formally launched on April 22 with a kick off meeting. Below is a tentative schedule:
● Mentor/Mentee registration opens: Saturday, 3/11/2023
● Registration closes: Saturday, 4/8/2023
● Program acceptance, notification emails sent: Saturday, 4/15/2023
● Kick-off event: Saturday, 4/22/2023
● Mentoring groups convene: May to October
● Graduation: Saturday, 11/11/2023
Please visit http://www.cabsweb.org/can/ to learn more about CAN program. If you are interested in becoming a mentor or mentee, please complete the forms below by midnight on Saturday, 4/8/2022.
● MENTOR registration:
● MENTEE application:

If you have any questions, please contact the chairs of the CAN Program Xiaojie Chen (xchenselleck@gmail.com) or Jack Zhu (jackningzhu@gmail.com)
Best regards,
Xiaojie Chen and Jack Zhu
Chairs of CAN program


(images from 2022 CABS CAN program events)
● MENTOR registration:
● MENTEE application:
Please use the link or QR code above to register as a mentor or apply as a mentee.
1633 Old Bayshore Hwy STE 280, Burlingame, CA 94010
You are cordially invited to attend 2023 Chinese New Year celebration party organized by the Chinese American Biopharmaceutical Society (CABS). Please come to enjoy music, singing, dancing, Guzheng (Chinese zither), martial arts and other entertainments performed by local Chinese dancers and artists, experience authentic Chinese foods, having fun solving riddles, prize draw and much more.
Please mark your calendar, and your early online registration will be greatly appreciated. We look forward to seeing you there and celebrating Year of the Rabbit together!

Senior Center, 266 Escuela Ave., Mountain View, 94040
The 2023 CABS Investor Forum has been successfully held on Jan 11, 2023, in the 2023 JP Morgan Healthcare Conference Week. This annual forum was resumed after a hiatus of three years due to the COVID pandemic. The Forum was held in a hybrid format for the first time. Nearly 140 attended the in-person event held in the San Francisco office of Morrison Foerster, and more than 60 people joined through Zoom meeting.

Attentive audience of the Forum
The Forum was kick started by Carrie Wang, the President of CABS, with her opening remarks. She introduced CABS to the audience and highlighted some of the CABS activities in 2022.

Carrie Wang, the President of CABS, giving opening remarks
The Forum consists of two panel discussions. The first panel was moderated by Janet Xiao, Partner of Morrison Foerster, and its theme was Trends, Opportunities, and Outlook for Biotech Investment. Five panelists brought in extensive and diverse backgrounds and insights into this panel discussion.

Moderator and panelists of the 1st Panel Discussion
Christian Grondahl, Co-Founder and CEO of SNIPR Biome, is a veteran entrepreneur. He has built and supported many successful companies, including taking one company, Zealand Pharma, to IPO. Grondahl suggested being in focus in 2023. He believes spending the minimum money a company needs and focusing on the core experiments are the ways to survive. He also emphasized making therapies cheaper in the long run with new technologies.
Jim Krenn, Partner of Morrison Foerster, shared his insights as a lawyer that terms in the investment agreement have shifted as deals are more favorable to investors. As a result, there is more emphasis on control terms in addition to economic terms. He suggested several routes for capital, including existing investors, debt, and licensing & partnership.
Arthur Kuan, CEO of CG Oncology, is a very young and successful entrepreneur of his age. Having raised $120M in a recent round, he said CG Oncology still needs to cut pipelines and be more focused. He suggested that entrepreneurs think in the way investors do: make sure the return and valuation of the company make sense to investors.
Cheni Kwok, Managing Partner and Founder of Linear Dreams, is a very experienced consultant who has served over 60 clients in the past ten years. She saw in the past fundraising was too easy, and companies were not “smart” enough to use money. For example, some companies may have generated insufficient clinical data. She suggested that even it may be a hard time for the majority, opportunities still exist. For example, now may be the best time to recruit as many companies are laying off top talents.
Sean Kendall, Partner of ARHC Venture, shared his opinion and observation from an investor’s standpoint. As innovation continues and research breaks through, the underlying philosophy for investment has not changed. Existing portfolio companies may take a longer time to revive. However, some companies ARCH met before may need money, which will be a good time for investors. ARCH invests in both problems solving-driven and opportunistic ways. It believes in people and may invest in the same people in different projects.
The 2nd panel was moderated by Alex J. Zhang, Managing Director of Lifespan Biolabs, and its theme was about Growing Opportunities and Strategies for Biopharma Companies in a Changing World. Again, we had panelists representing a diverse spectrum of biotechs: John Adamou, CBO of CSPC Pharmaceutical Group; Oliver Kong, CMO of IASO Biotherapeutics; Neela Patel, CBO of Bonum Therapeutics; Eugene Wang, Founder and CEO of Help Therapeutics; and Allen Jiang, CSO of Xtalpi.

Moderator and panelists of the 2nd Panel Discussion
The panelists discussed the keys to surviving and thriving under the current stock market and geopolitical environment, partnering trends, and promising technologies. The panelists expressed an opinion similar to that of the previous panel: companies over-raised and over-built in the past few years. It is a challenging environment but not a terrible market, as MNCs are still spending. The panelists all noted and echoed the following topics: managing cash flow, driving down costs through automation and technology innovation, creating value, and emphasizing the importance of assets rather than platforms.
Patel brought up a fascinating but rarely discussed topic. When Roche acquired Good Therapeutics, she decided to spin off the technology platform to Bonum and left the asset in Good to be tax efficient for its stakeholders. This tax strategy helped investors to make more money and invest in Bonum Therapeutics confidently.
Adamou mentioned two ecosystems relevant to all biopharma in China: Global to China and China to Global. China has an extensive healthcare market challenging to navigate. Companies like CSPC have vast on-the-ground sales teams and manufacturing capabilities. It is looking for new therapeutics to complement its existing generic catalogs. On the other hand, CSPC doesn’t have a solid ability to commercialize drugs in ex-China markets as MNCs do. It is also looking to out-license drugs and find partners in ex-China rights that it is developing.
Both panels stirred warm discussions in the audience.


Animated Q&A
Same as the past Forums, complimentary breakfast and lunch were generously provided by Morrison Foerster. The times at meals and coffee break provided ample opportunities for attendees to network and interact with themselves and the moderators/panelists. The successful continuation of the Forum is yet another example attesting the vision and mission of CABS to connect and serve the biopharmaceutical community.

On January 14, 2023, CABS celebrated the first Career Advisory Network (CAN) graduating class post COVID.

CAN is a mentoring program in the Career and Business Development (BCD) group in CABS. It is a direct one to one pairing mentorship program to guide mentees in their careers including business development, traditional scientific career paths in both academia and industry, and entrepreneurship. This is a free program for CABS members. The mentorship program is only made successfully through hard work, dedication, persistence, and service of the mentors who in their busy schedules carve out time to guide the mentees.
The graduation opened networking opportunities and lite snacks. The main event was the sharing of both mentors and mentees shared their reflections, experiences, and overall learnings.


Many mentors share that it was not only an opportunity for them to give back to the next generation, but also an opportunity for them to continue to learn from their mentees as well. There were many key takeaways and lessons.

Mentees range from early careers to late career stages to newly transitioned career paths. They were overjoyed to have been paired with a mentor to guide them in their career growth, navigating strategically, and communicating successfully for the next phase of achieving their career goals. Many expressed continuances of the program in 2023 as well as some mentees opening to transition to be a mentor.

Gifts of appreciation were provided to both mentors and mentees. The closing of the celebration included lunch at HL Peninsula, Burlingame where many conversations between mentors and mentees continued to grow as part of the career networking opportunity created by the CAN program.

BCD looks forward to hosting another CAN program in 2023.
Chinese American Bio/Pharmaceutical Society (CABS) is pleased to announce that 2023 CABS Investor Forum will be held on January 11th of 2023 in conjunction with JP Morgan Healthcare Conference. In the post-COVID era, pharmaceutical innovation, medical technology and healthcare continue to be the major areas of licensing and venture capital investment globally. This forum will feature two panel discussions joined by investors, lawyers, business development executives, and entrepreneurs. The topics will focus on the investment trends, licensing and strategic considerations, as well as new opportunities and challenges in life sciences.
This is a hybrid event FREE to both in-person attendees and virtual attendees.
FOR IN-PERSON ATTENDEES
Kindly note that the registration through this website is for in-person attendees ONLY. If you register, please attend. There are limited seats at Morrison & Foerster. Each year many people are on the waiting list of CABS Investor Forum.
Registration and a photo ID are required to enter the venue (there will be a security check). Morrison & Foerster requires everyone entering the office to be fully vaccinated and masks are optional but strongly recommended.
Registration deadline for onsite attendees is 3PM, Jan 5, 2023 (Wednesday, Pacific time). First come, First serve. We recommend that you register ASAP.
FOR VIRTUAL ATTENDEES
If you are interested in virtually attending this event, please register via the following link. The registration is also FREE.
https://us06web.zoom.us/webinar/register/WN_IQ5TDr49QoSgRMJWkYm1Cg
[The above link is for VIRTUAL REGISTRATION ONLY]
AGENDA
8:00 AM –9:00 AM Registration, Breakfast and Networking
9:00 AM –9:10 AM Welcome Remarks by Carrie Wang, President, CABS
9:10 AM –10:10 AM Panel Discussion #1: Trends, Opportunities, and Outlook for Biotech Investment
Moderator: Janet Xiao, Partner, Morrison Foerster
Panelists: Christian Grøndahl, Co-founder and CEO, SNIPR Biome
Sean Kendall, Partner, ARCH Venture
Jim Krenn, Partner, Morrison Foerster
Arthur Kuan, CEO, CG Oncology
Cheni Kwok, Managing Partner and Founder, Linear Dreams
10:10 AM –10:30 AM Coffee Break
10:30 AM –11:30 AM Panel Discussion #2: Growing Opportunities and Strategies for Biopharma Companies in a Changing World
Moderator: Alex J. Zhang, Managing Director, Lifespan BioLabs
Panelists: John Adamou, CBO, CSPC Pharmaceutical Group
Alan Yide Jiang, CSO, XTalPi
Oliver Kong, CMO, IASO BioTherapeutics
Neela Patel, CBO, Bonum Therapeutics
Eugene Jiaxian Wang, Founder and CEO, Help Therapeutics
Michelle Xia, Founder, Chairwoman, President and CEO, Akeso Biopharma
11:30 AM –1:00 PM Lunch and Networking
Introduction of Moderator and Panelists
Panel #1

Janet Xiao, PhD, JD, Partner, Head of firm's China Life Sciences Group, Morrison Foerster LLP
Janet Xiao is the head of the firm’s China Life Sciences Group, and is among the very few IP attorneys in the world who are equipped with solid skills in global strategic IP management and knowledge about IP issues unique to China and Chinese clients. She focuses her practice on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies with clients ranging from large multinational biopharmaceutical companies. She advises on patent and IP due diligence matters relating to various technologies, including antibody therapeutics, cell therapeutics, nanomedicine, gene therapy, drug delivery systems, diagnostics, and nutraceuticals.
Janet works extensively in performing IP due diligence reviews in the contexts of cross-border VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. She has led hundreds of IP due diligence projects, helping some of the world’s most-sophisticated biopharmaceutical companies and investors assess potential risks and devise preemptive risk-management strategies for their product development and investments. She is noted for her in-depth knowledge of patent landscapes in the fields of cancer immunotherapy, gene editing, next-generation sequencing, liquid biopsy, antibody manufacturing, and medical devices.
Christian Grøndahl, PhD, Co-founder and CEO, SNIPR Biome
Christian Grøndahl received his Doctor of Medical Science, PhD and Doctor of Veterinary Medicine degrees from University of Copenhagen and his MBA from IMD, University of Lausanne, Switzerland. Christian worked as a veterinary physician/surgeon and assistant professor before moving to pharma and biotech where he has spent the last 20+ years in R&D, Corporate and Executive roles.
Before co-founding SNIPR Biome, he served as the CEO of Kymab Ltd. in Cambridge, UK, and was partner in the investment arm of The Wellcome Trust, London, Syncona LLP. Christian has been part of starting several companies. In addition to SNIPR Biome, this includes Freeline Therapeutics, Gyroscope Therapeutics, Quadrucept Bio Ltd., and Folium Food Science. Christian served as Executive Vice President, CSO and Head of R&D in Zealand Pharma and was part of taking the company through an IPO in 2010. Christian served in VP and Corporate VP roles with Novo Nordisk, heading up Cancer and Inflammation, Women’s Health, eClinical Development and Corporate Development and Strategy. Christian is the co-inventor on 20+ patents and patent applications licensed to public and private companies.
Presently, Christian serves as the Co-founder and CEO, SNIPR Biome APS (Copenhagen, Denmark), Co-founder and Chairman, QUADRUCEPT BIO Ltd. (Cambridge, UK), Co-founder and Chairman of the Board, FOLIUM SCIENCES Ltd. (Cambridge, UK), Chairman of the Board in TUBULIS GmbH, (Munich, Germany).
Sean Kendall, Partner, ARCH Venture
Sean Kendall focuses on identifying and evaluating new life science technologies and provides operating assistance to early-stage portfolio companies. He contributed to the formation and funding of Vizgen, AIRNA, Scale Biosciences, and Lightcast Discovery, and supported early efforts for Encodia, Singleron Biotechnologies, and Twist Bioscience (TWST). He is an active board member at Encodia and AIRNA, and sits as a board observer at Vizgen and others, having previously served as an observer at Twist. Mr. Kendall also supports ARCH Strategic Partnerships, where he has encouraged breakthrough innovations in electronics, sustainable materials, energy, and more via corporate partnering and investment.
A chemical engineer and synthetic biologist by training, Mr. Kendall was an early employee with LS9, Inc. (acquired by Genomatica), where he developed an automated experimentation and analysis platform for their synthetic biology platform. He next scaled commercial processes for Cobalt Technologies. Mr. Kendall is a recipient of the President’s Green Chemistry Challenge Award. He holds a B.S. and M.S. in Chemical Engineering from Stanford University, where he worked with Jim Swartz on cell-free protein synthesis, and an M.B.A. from the University of Chicago.
Jim Krenn, JD, Partner, Morrison & Foerster LLP
Jim Krenn has extensive experience representing private and public companies and investors in significant business transactions from formation through liquidity. His practice focuses on mergers and acquisitions, venture capital financings, and emerging company counseling for clients in a broad range of industries, including biopharmaceuticals, medical devices, artificial intelligence, and agtech.
Jim advises clients on private and public acquisitions on both the buy-side and sell-side across numerous transaction structures. He also counsels emerging and established life sciences and technology companies in equity financings and general corporate matters, and angel, corporate, and venture capital investors in investments in such companies. He has represented buyers and sellers in acquisitions and divestitures ranging from the low millions to over $1 billion as well as clients in over $2 billion in equity and debt financings ranging from seed rounds to $100 million-plus later-stage rounds.
Arthur Kuan, MS, CEO, CG Oncology
Arthur is Chief Executive Officer on the Board at CG Oncology. Having lost his father to cancer this is personal for him. Arthur was recognized in August 2022 by Business Insider’s 30 People Under 40 Who Are Transforming Healthcare. He was named a 2020 Forbes 30 Under 30 featured honoree in healthcare. At Moffitt Cancer Center, Arthur serves on the IP Commercialization Strategy Committee. Arthur is a Founding Member of Ally Bridge Group, a global healthcare-focused investment platform, and played an active role in managing the fund’s portfolio of companies. Previously, Arthur was a member of Themes Investment Partners, a Private Equity fund where he focused on healthcare and biotech investment opportunities. He began his career in an operational role at Dinova Capital, a MedTech incubator fund, focusing on cross-border technology transfer and new company formation. Arthur received his M.S. in Biotechnology from the Johns Hopkins University and his B.A. in Biology from the University of Pennsylvania.Cheni Kwok PhD, CLP, Managing Partner and Founder, Linear Dreams LLC
Dr. Cheni Kwok is a senior biopharmaceutical executive with broad operational expertise who has executed over 200 transactions including M&A, strategic partnerships, licensing, divestitures, spin-offs and project financing. Dr. Kwok is the Managing Partner and Founder of Linear Dreams LLC, a management consultancy for the life sciences industry. The firm’s engagements include a broad range of business and corporate development activities for 66 biopharmaceuticals companies, contract research & non-profit organizations, research institutes and investors in USA, Europe, China, Taiwan, Korea and Singapore. Prior to founding Linear Dreams, Dr. Kwok served as Senior Vice President, Corporate Development at Poniard Pharmaceuticals Inc., Director of Business Development at Celera Genomics Inc., Associate Director of Business Development at Exelixis Inc.. and in various research management, technology assessment and alliance management roles at SmithKline Beecham plc (now GlaxoSmithKline plc). Dr. Kwok received a bachelor's degree with first class honors in biotechnology from Imperial College London, UK, a Ph.D. in human molecular genetics from the University of Cambridge, UK and has earned the Certified Licensing Professional credential. At present, Dr. Kwok is serving on the Board of Directors of the Chinese-American Biopharmaceutical Society.
Panel #2

Alex J. Zhang, PhD, MBA, Managing Director of Lifespan BioLabs
Dr. Zhang is Managing Director of Lifespan BioLabs, a life science accelerator and investor based in San Francisco Bay Area. He was the Chief Scientific Officer of Hanhai Holdings Group, and the CEO of Hanhai Silicon Valley, Inc., an early-stage investor and cross-border incubator for life sciences and frontier tech startups based in San Francisco Bay Area. Prior to Hanhai, Alex was the Co-founder and Managing Partner of Enverest, LLC., a Silicon Valley based innovation solutions and investment advisory firm, with branch offices in China and Singapore. Prior to founding Enverest, Dr. Zhang spent over four years at Thermo Fisher Scientific, where he was responsible for 4 business development deals exceeding $10 M, and played a key role in several billion dollar acquisitions in IVD and MedTech areas. From 2001 to 2009, Dr. Zhang was a Senior Scientist at Tularik Inc. (acquired by Amgen in 2004), where he led drug discovery endeavors in oncology, cardiovascular and metabolic diseases therapeutic areas. Over the past decade, Dr. Zhang has been advising a number of successful biotech, MedTech and digital health startups and VC firms based in Silicon Valley, including establishing and seed-funding of an iPSC cell therapy startup.Dr. Zhang is a Board Member of the Chinese America BioPharmaceutical Society (CABS), a highly influential association with more than 4000 members and subscribers in the life sciences industry. He served a number of leadership roles in the Executive Council of CABS, including as the President in 2017-18. CABS is the largest and most active Chinese biopharmaceutical association in Northern America. Dr. Zhang was the Chair of the Organization Committee for 2017 BioPacific Conference and 2018 CABS Investor Forum.
Dr. Zhang received MBA degree at Cornell University, PhD in Organic and Analytical Chemistry at Texas A&M University, and BS in Chemistry at Shandong University. During his graduate research, he focused on the design and synthesis of therapeutic peptoids, as well as biological mass spectrometry. His research has led to publication of 17 peer reviewed articles and 4 patents.
John Adamou, MBA, MS, CBO, CSPC Pharmaceutical Group US
John Adamou is Chief Business Officer, CSPC Pharmaceutical Group US. John has leadership responsibility for the strategic partnering and business development activities across various therapeutic areas. John has over 25 years of business development, strategic transactions, and scientific experience in the biotechnology and pharmaceutical industry at CSPC, BeiGene, Boehringer Ingelheim and Medlmmune. John has an established track-record of signed business development deals across a broad array of deal structures in various therapeutic areas in North America, Europe, and Asia.
Earlier in his career John held various research roles at MedImmune, SmithKline Beecham, and Allelix Biopharmaceuticals. John holds BS and MS degrees in Molecular Biology and Genetics from the University of Guelph, Canada, and an MBA.
Yide Alan Jiang, MD, PhD, CSO, XtalPi Inc.
Dr. Jiang is Chief Strategy Officer, Director of the Board of XtalPi, where he bears primary responsibility for the company’s strategy development including identification of growth opportunities, strategic planning and execution. XtaiPi is a company integrating quantum physics theory, AI and cloud computing positioned to be the next generation industry model for biopharmceutical and material Research & Development. Dr Jiang joined Xtalpi with over twenty years of scientific and research management experience, most of it gained in positions of increasing responsibility at Genzyme and then Sanofi-Genzyme. Prior to joining XtalPi, he was the director of Asia R&D Strategy of the Sanofi-Genzyme R&D Center and in that capacity, he was responsible for the development of Genzyme Asia/China R&D strategy and led cross-functional R&D external collaborations and projects in Asia.
Oliver Kong, MD, PhD, CMO and EVP, IASO Biotherapeutics
Dr. Kong joined IASO Biotherapeutics in September 2022, and is currently the company’s Chief Medical Officer and Executive Vice President.
Dr. Kong holds a doctor's degree from Shanghai Medical College of Fudan University and a master's degree of Science in Molecular Immunology from University of Nebraska Medical Center.
Dr. Kong has more than 25 years of experience in the pharmaceutical industry in clinical development and medical affairs in various diseases or treatment fields such as oncology, autoimmune diseases and infectious diseases. Before joining IASO Biotherapeutics, he served as the Chief Medical Officer and Corporate Vice President of Qilu Pharmaceuticals, the vice president of Clinical Oncology of Hengrui Therapeutics, the Senior Global Medical Leader of Global Medical Affairs of Novartis Oncology and the Medical Director of US Medical Affairs of Bayer HealthCare Pharmaceuticals.
Neela Patel, PhD, CBO, Bonum Therapeutics
Dr. Neela Patel is a scientist and business development executive with more than 25 years of leadership experience in drug discovery & development, project & portfolio management, and pipeline development. She currently serves as Chief Business Officer at Bonum Therapeutics. Previously, as Chief Business Development Officer at Good Therapeutics she led the acquisition of Good by Roche for an upfront payment of $250M, and in parallel led the spinout of Bonum from Good in 2022. As Executive Director of Corporate Development at Seagen, she played an integral role in deal sourcing and execution of collaborations, licenses, and acquisitions to build the pipeline. Previously, Dr. Patel’s sourcing and diligence work at AbbVie in resulted in more than 25 executed deals. Dr. Patel spent the first 16 years of her career in drug discovery with positions of increasing responsibility, advancing small molecules and biologics from target ID and validation through IND filing at Poniard Pharmaceuticals, Genentech, SUGEN/Pharmacia, and Roche Bioscience. Over the course of her career, she has advised a variety of startup companies on BD and corporate matters.
Dr. Patel completed her post-doctoral training at the DNAX Research Institute after receiving her PhD in Molecular Biology from UCLA. She graduated Phi Beta Kappa from Stanford with a B.S. in Biology and a B.A. in Humanities with Honors.
Eugene J. Wang, PhD, CEO, Help Thera-X Limited
Dr. Eugene J. WANG is the CEO of Help Thera-X Limited, which he founded in 2016. A gold medalist at University, Dr. WANG received his research fellowship training in Mount Sinai School of Medicine and worked as a clinical cardiologist at The First Affiliated Hospital with Nanjing Medical University (Jiangsu Province Hospital, ranked in Top 20 in China) after earning his PhD from the University of Hong Kong and MD from Capital Medical University. A research scientist in Stem Cell and Regenerative Medicine,
Dr. WANG strongly believes that innovative technology in induced pluripotent stem cells (iPSC) development is essential to solve degenerative problems caused by aging-related intractable diseases. Under Dr. WANG’s leadership, Help Thera-X has grown to become a globally clinical-stage biopharmaceutical company dedicated to the development of first-in-class iPSC-derived cell therapeutics for heart failure and neurodegenerative diseases. Over the past 6 years, Dr. Wang has led the team to complete world’s First-in-Human trial of induced pluripotent stem cellsbased heart repair, which was reported by Nature in 2020. A serial entrepreneur with a passion for innovative ideas, Dr. WANG is an industrial leader in automatically robotic cell manufacturing system integrated machine learning and data analytics techniques. The multi-scale and high-performing industrial cell production operating to cGMP standards is the first in the world to meet the real-world clinical demand of iPSC-CMs in purity and amount for cell transplantation.
Dr. WANG also spearheads in shaping cardio-regeneration education and policy through his association with several committees in China such as: research fellowship in Science & Technology Institute of National Health Commission of China, Chair PI of China iPSC Model Bank for Intractable Diseases, Executive Director of China Stem Cell Industry Alliance, etc. Dr. WANG has been honored by prestigious awards including State’s Key Project of R&D Plan, National Natural Science Foundation, Leadership of Innovation Award of the Year, and Best Entrepreneur of Jiangsu Province.
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105

Instructions for Sponsors
Greetings!
Welcome to 2022 BioPacific Conference!
Please find the information below for setting up the exhibition booth.
Exhibition Hall: Convene lobby and Inspire room #1, #2, #3 (Located at second floor of Marriott)
Exhibition booth includes:
One exhibition table (size: 6’ x 30’’) and two chairs
Exhibition hours: 8:00 am - 7:00 pm
Booth set up time: 6:00 am - 8:00 am
Wrap up time: 6:00 pm - 7:00 pm
Exhibition table assignment:
Please see below for exhibition hall layout and your table assignment.
Notes:
- Please register all the representatives from your company by November 8, 2022 so we can order enough lunch.
- Exhibition hall will be open at 6:00 am. Please come on time to set up the booth. The attendee’s registration starts from 8:00 am. You can pick up your name badge at the Sponsors’ registration table.
- It is highly recommended to have at least one of your company representatives stay at your booth from 8:00 am-5:00 pm (you can stay until 7pm, if you like) to introduce your products/services.
- Parking in the hotel parking lot is complimentary so no stamp is needed.
- Free coffee and lunch will be provided.
- All sponsors are welcome to join the post-conference reception (5:00pm-6:00pm) and China Night session (5:30pm-8:00pm).
Please direct any questions to:
Jessica Sun & Weixing Chen
Co-chairs, Alliance Management Committee, CABS
E-mail: fundraising@cabsweb.org
Thanks to our Sponsors!
San Mateo Marriott San Francisco Airport, 1770 South Amphlett Blvd, San Mateo, CA 94402

CABS 2022 BioPacific Conference
Resilience and Ingenuity -- Embracing Opportunities in a New Normal
CABS 2022 BioPac Conference programBook
CABS is pleased to host its 23rd annual conference.
Date/Time: 8:00AM - 8:00PM PT on Saturday, Nov 12, 2022.
Location: San Mateo Marriott SFO, 1770 S Amphlett Blvd, San Mateo, CA 94402
Limited sponsorship opportunities available. Please contact fundraising@cabsweb.org.
While everyday life is starting to normalize in many parts of the world despite ongoing COVID-19, economic and supply chain turbulences present both new challenges and opportunities for the life science and biopharmaceutical industries. This year’s conference will showcase success stories and best practices for innovation and improving the resilience of organizations. We expect to attract more than 600 attendees globally from the life science and biopharmaceutical industries. The conference features 2 keynote speeches, 7 podium presentations and 2 panel discussions, with major topics highlighted below.
• Best practices for small molecule drug discovery
• A new class of oncology drug targets
• Winner of the 2022 CABS K. Fong Award in Life Sciences
• Success stories from pharma entrepreneurs
• Building a robust drug candidate pipeline
• Pharma partnering and investment strategies
• New technologies for studying protein structures
• Targeting an oncoprotein previously thought to be undruggable
• Leveraging digital technologies to accelerate medical innovation
• China-US cross-border collaboration with large pharmaceutical companies
Confirmed Speakers


Turning Point Therapeutics was successfully acquired by Bristol Myers Squibb for $4.1 billion in 2022.




Agenda
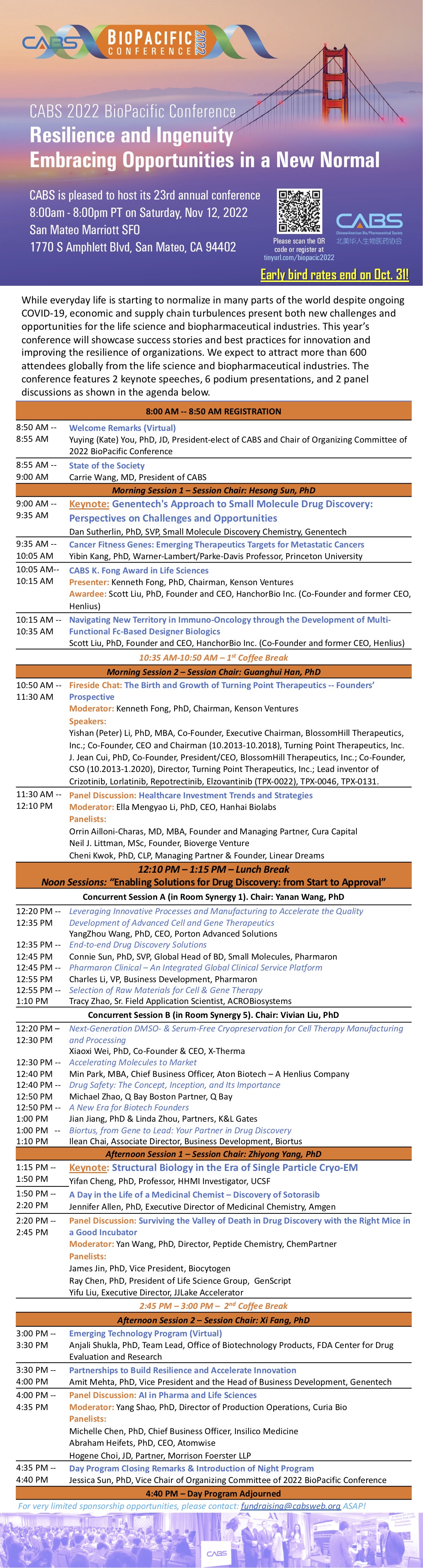

Registration Fees
Registration online as early as possible is highly recommended.
The registration on November 12, 2022 and on-site will be $150.
| CABS Member |
Early Bird Registration(by 10/31/2022) |
Regular Registration (11/1/2022- 11/10/2022) |
| General | $60 | $100 |
| Academic | $40 Please use your institution email (.edu) |
$80 Please use your institution email (.edu) |
| Non-Member* | Early Bird Registration (by 10/31/2022) | Regular Registration (11/1/2022- 11/10/2022) |
| General | $90 | $130 |
| Academic | $70 Please use your institution email (.edu) |
$110 Please use your institution email (.edu) |
* Refreshments, lunch are included.
* Join CABS today ($30 for one year membership) to save on registration fee: http://www.cabsweb.org/members/
* Please contact info@cabsweb.org, if there are any questions about registration.
Thanks to our 2022 BioPacific Sponsors!


Thinking about becoming a sponsor of the BioPacific Conference? Please contact fundraising@cabsweb.org . Thank you very much!
San Mateo Marriott San Francisco Airport, 1770 South Amphlett Blvd, San Mateo, CA 94402

CABS 2022 BioPacific Conference
Resilience and Ingenuity -- Embracing Opportunities in a New Normal
CABS 2022 BioPac Conference programBook
CABS is pleased to host its 23rd annual conference.
Date/Time: 8:00AM - 8:00PM PT on Saturday, Nov 12, 2022.
Location: San Mateo Marriott SFO, 1770 S Amphlett Blvd, San Mateo, CA 94402

The 2022 Biopacific Conference, also known as the 23rd Chinese American BioPharmaceutical Society (CABS) Annual Conference, was held on November 12th, 2022 at San Mateo Marriott San Francisco Airport, California. As our first in-person conference since the COVID-19 pandemic, it had 42 distinguished speakers/panelists/moderators, 51 sponsors, and attracted more than 600 attendees. The amazing success would have not been possible without the contributions and participation of all the speakers, sponsors, attendees, organizing committee members and volunteers.

The theme of the Conference was “Resilience and Ingenuity, Embracing Opportunities in a New Normal.” The conference included 3 keynote talks, 1 K. Fong Award speech,1 fireside chat, 4 scientific speeches, 5 panel discussions, and 9 breakout room presentations, all by industry and academic leaders. These exciting talks and panels were grouped into 2 morning sessions, 2 noon sessions, 2 afternoon sessions, and the inaugural China Night session.

To commence the Conference, Dr. Kate You, President-Elect of CABS and Organizing Committee Chair of the Conference, gave warm welcome remarks and an overview of the agenda. Dr. Carrie Wang, President of CABS, then gave the State of the Society speech, highlighting the activities, growth, and achievements of CABS in the past year.

Morning Session 1 (Chair: Dr. Hesong Sun)

Dr. Dan Sutherlin, Senior Vice President of Small Molecule Drug Discovery at Genentech, presented the first keynote speech and first discussed the challenges in drug discovery, using Crohn's disease as an example. He went on with three vignettes to delineate some of Genentech's approaches to tackle the challenges: using variations of protein degradation technology to expand druggable space; using Cryo-EM to uncover structures to guide the optimization of drug candidates; and using AI to accelerate the pace of drug discovery process. Dr. Yibin Kang, professor at Princeton University, described a new class of cancer fitness genes that are critical for cancer metastasis but not for normal tissues. Thus, inhibiting these genes resulted in highly selective toxicity against cancer cells. Dr. Kang’s lab discovered metadherin as a poor-prognosis marker in a high throughput screening study, and validated the importance of this protein in tumor growth and metastasis in animal models.

Following the two exciting talks, Dr. Kenneth Fong presented the 2022 CABS K. Fong Award to Dr. Scott Liu, founder and CEO of HanchorBio Inc, Co-Founder and former CEO of Henlius.
In his acceptance speech, Dr. Scott Liu pointed out that most monovalent and bivalent biologic drug candidates failed in clinical trials as they cannot block the interactions between the target protein and multiple ligands all at once. HanchoBio achieved the goal of simultaneous targeting with its novel Fc-Based Designer Biologics (FBDB™) platform. HanchorBio has also overcome the manufacturing challenges associated with producing such novel large molecules and can produce the designer molecules at mg/L concentrations. Multiple FBDB™ in HanchorBio’s pipeline showed promising in vitro and in vivo efficacy against lymphoma and solid tumors such as lung cancer and colorectal cancer.
Morning Session 2 (Chair: Dr. Guanghui Han)

The Fireside Chat within the session was moderated by Dr. Kenneth Fong with two outstanding entrepreneurs, Drs. J. Jean Cui and Yishan (Peter) Li, the Co-Founders of Turning Point Therapeutics. They talked about how the company got started, the key ingredients for its success, and shared their experiences with the audience. Dr. Kenneth Fong’s moderation was full of humor, and the entire Fireside Chat was joyous.
The following panel discussion of the session, with Dr. Ella Li as moderator, and Drs. Orrin Ailloni-Charas, Cheni Kwok, and Mr. Neil Littman as panelists, discussed the healthcare innovations from the perspective of investors and strategists, including their own decision-making process in healthcare investment and corporate strategy, the challenge and hurdle they’ve ever had, their thoughts on the most promising healthcare trend in next 10 years, and fun discussion about the most overhyped and underhyped technologies in the healthcare industry.
Noon Sessions (Chairs: Drs. Yanan Wang and Vivian Liu)

The two parallel noon sessions consisted of 9 lectures that showcased better tools and approaches - from manufacturing to patients' ends - for drug discovery. Dr. YangZhou Wang, CEO of Porton Advanced Solutions, emphasized the importance of a reliable CDMO with both manufacturing capabilities and sufficient capacity to the success of gene and cell therapy (GCT) products. Dr. Connie Sun, SVP and Global Head of BD, Small Molecules at Pharmaron, described her company’s integrated drug discovery services team that leads small molecule projects from hit identification to candidate selection. Mr. Charles Li, VP of BD at Pharmaron, introduced Pharmaron’s integrated platform of global clinical services that provide a one-stop shop for its sponsors in both US and China. Tracy Zhao, Sr. Scientist at ACROBiosystems, described how the selection of raw materials can have a huge impact on the success of GCT products. Dr. Xiaoxi Wei, co-founder and CEO of X-Therma Inc, introduced the application of convergent biomimetic nanoscience to solve the limitation of off-the-shelf products for advanced regenerative medicine. Min Park, Chief Business Officer at Aton Biotech – A Henlius Company, shared how to deploy smart platform strategies to accelerate molecules to markets. Dr. Michael Zhao, Q Bay Boston Partner, emphasized the importance of drug safety and discussed the role of investors in maintaining efficient systems for drug safety and good pharmacovigilance practice. Linda Zhou and Thomas Anderton, Partners of K&L Gates, introduced their career pathways and shared previous successful cases in the pharmaceutical field. Ilean Chai, Associate Director of BD at Biortus, shared a few examples of gene-to-lead generation pipeline in the context of fragment screening and SAR for drug discovery.
Afternoon Session 1 (Chair: Dr. Zhiyong Yang)

This session consisted of two talks and one panel discussion. Dr. Yifan Cheng, a member of National Academy of Sciences and American Academy of Arts and Sciences, an HHMI investigator, and a professor at UCSF, opened the session with his keynote speech titled “Structural Biology in the Era of Single Particle Cryo-EM.” Dr. Cheng first gave a historical overview of the development of the Cryo-EM technology, then shared vignettes in using the technology to solve structures of protein complexes, identify novel conformations of the spike proteins of SARS-CoV-2 with the assistance of different specific antibodies. Next, Dr. Jennifer Allen, Executive Director of Research at Amgen, gave a talk titled “A Day in the Life of a Medicinal Chemist – Discovery of Sotorasib.” In Dr. Allen’s eyes, every atom matters. She showed us how her team repeatedly applied the Structure-Activity-Relationships paradigm to optimize pharmacological properties of drug candidates that ultimately led to the discovery of Sotorasib, an FDA-approved KRAS G12C inhibitor.
This session ended with a panel discussion with Dr. Yan Wang as moderator, and Drs. James Jin, Ray Chen, and Mr. Yifu Liu as panelists. The panel gave an in-depth discussion on the role of incubator/CRO in the drug discovery process. First, the panelists shared their opinions on how to select the right incubator/CRO among a few hundred candidates in the market. Then they explained their annual budgeting for investing in new technology, reagents, and platforms to support drug discovery. Finally, they discussed how they tried to balance the investment in research and the need to achieve their revenue goal.
Afternoon Session 2 (Chair: Dr. Xi Fang)

Starting this session, Dr. Anjali Shukla, Lead of FDA Emerging Technology Team (ETT), introduced the FDA Emerging Technology Program. In this program, pharmaceutical companies can, prior to the regulatory submission, submit questions and proposals about the use of specific emerging technology to the FDA ETT group, which includes relevant representation from all FDA pharmaceutical quality functions. The ETT works in partnership with relevant pharmaceutical quality offices and assumes a (co-)leadership role for cross-functional quality assessment (including review and on-site facility evaluation or inspection) for submissions involving emerging technology. Dr. Amit Mehta, Vice President and the Head of BD at Genentech, provided an overview of Genentech’s approach to evaluate external innovation, strategic focus areas, and recent partnerships. Dr. Mehta also highlighted case studies for Asian-Pacific partnership and execution strategies.
The panel discussion “AI in Pharma and Life Science” of this session was joined with 3 global experts, Drs. Yang Shao, Abraham Heifets, Michelle Chen, that led the effort of applying AI to innovations in pharma and life sciences. Three critical topics were discussed in depth: the myths and challenges people may face when we try to introduce more and more AI applications to our industry; the added values AI would bring to our industry; and the latest FDA regulation regarding AI applications in our industry and its current and future impact on the industry.

The day program ended with closing remarks by Dr. Jessica Sun, Vice Chair of the Organizing Committee of the 2022 Biopacific Conference. Her remark “Today we are all volunteers!” resonated with the audience with a big round of applause.
China Night

This program is special at least in twofold: first, it was the first night session in the history of the Conference; second, it was the first time the Conference has trans-Pacific parallel sessions connected by internet. The program was the result of the close partnership among CABS, Fosun Pharma USA, BayHelix, and Hanhai BioLabs. China Night included one keynote speech and one panel discussion in Shanghai, and another panel discussion in San Mateo. The night program began with the welcome remarks by Mr. Deyong Wen, CEO of Fosun Pharmaceutical; and Dr. Carrie Wang, President of CABS.

During the keynote talk, Mr. Yuanyuan Qi, COO of Fosun Kite, shared the history of his company, from the company buildup, technology development, clinical registration, to the final commercialization of its first license-in. a CAR-T product. Mr. Qi elaborated how Fosun Kite’s successful business strategy and strong communications with the regulatory agency managed to make the first commercialization of a cell therapy product in China.
The first panel discussion (in Shanghai), with Dr. Wei Wu as moderator, and Drs. Guoliang Yu, Jinfu Yang, Dajun Yang, and Jun Zhu as panelists, had an insightful sharing on accelerating globalization by expanding the Chinese Biopharma Innovative Ecosystem. They advised decision makers to design up-to-date and long-term strategies and adjusting them continually during the implementation process. The second panel (in San Mateo), moderated by Dr. Alex Zhang and joined by panelists Drs. David Shen, George Wu, Dandan Dong, and Janice Zang, had an insightful discussion on the innovations of US-based biotechs and new developments in therapeutics. They shared entrepreneurial experience in the field of peptide technology, drug delivery, and target therapy. Innovative mechanisms and investment strategies in the US were also discussed in great depth.

To conclude, we would like to express our humble gratitude again to all speakers, sponsors, attendees, organizing committee and volunteers. It was your unwavering support and unquenchable passion for CABS and the biopharmaceutical community that rendered great success to the Conference. We look forward to seeing you in future events.
Thanks to our 2022 BioPacific Sponsors!


Thinking about becoming a sponsor of the BioPacific Conference? Please contact fundraising@cabsweb.org . Thank you very much!
San Mateo Marriott San Francisco Airport, 1770 South Amphlett Blvd, San Mateo, CA 94402
ChinaBio® Partnering Forum 2022
Beijing and Digital
Nov 8-9, 2022 Beijing
Nov 10-11, 2022 Online
ChinaBio® Partnering Forum is the largest and most productive life science partnering event in China. To be held as a 4-day hybrid event; 2 days in-person, 2 days virtually, November 8-11, 2022, the conference will bring together biotech and pharma leaders from around the world along with hundreds of China-based developers of novel technologies for four days of successful partnering.
ChinaBio® Partnering Forum 2021 attracted 1,000+ delegates from over 500 companies and 27 countries, making it a must-attend global event for companies interested in developing cross-border partnerships, both inside and outside of China. The 2022 edition promises to be even better with the added ability to attend in-person and virtually, with top notch attendance from pharma and biotech companies and investors, as well as leading executives from China’s fast-growing life sciences hubs and researchers from China's top universities and institutes.
The conference also features EBD Group’s gold-standard partnering platform, partneringONE®, enabling delegates to efficiently identify, meet and network with companies from across the life science value chain in a virtual format, with each meeting prescheduled with its own video conferencing link. In addition to partnering meetings, the event will feature a blend of live and on-demand program sessions, company presentations, table-top exhibits, company showcases, and networking sessions.
CABS subscribers will enjoy 20% registration discount off the regular rate using promo code ‘CABS20’.
北京和虚拟会议
2022年11月8-11日
ChinaBio合作论坛是中国最大最富成效的国际性生命科学合作会议,将于2022年11月8-11日通过线下现场和线上虚拟的组合方式举行。此次会议将汇聚来自世界各地的生物技术和制药业领袖以及数百名中国新技术研发企业,进行为期四天的成功合作互动。去年,会议吸引了来自 27 个国家 500 多家公司的 1,000 多名代表,使其成为有意在中国境内外发展跨境合作关系的公司必须参加的全球盛会。除一对一合作会议之外,本会议还包括现场和点播观看的会议演讲、公司路演、桌面展览、公司展示以及各种社交环节。
CABS会员凭优惠代码CABS20,享受原价基础上8折注册优惠!
注册事宜:
Jean Meng
电子邮件: jean.meng@chinabio.com
In the USA
Yesenia Cortes
EBD Group
(212) 600-3448
ycortes@ebdgroup.com
In Europe
Philipp Dormeier
EBD Group
+49 89 23 88 756 0
hybrid (Beijing and Online)
-
Smart Partnering System (www.cabs-system.com)
We are very pleased to launch an Integrated Partnering System to help our members to find new business connections and post/find job opportunities. You are encouraged to post business demand and jobs on our new Smart Partnering System before Nov 12, 2022 BioPacific Conference to increase the possibilities of business developments and hirings.All the posts are free.
Please go to www.cabs-system.com to use CABS Smart Partnering System, on which you can:
- Post and manage your company’s business demands and job openings;
- Search and explore other companies’ business demands with multilevel fine-tuned filters, which help people
- quickly locate the demands;
- Search and explore job openings.
You can find tutorials about how to use this system at www.cabs-system.com/how.
For the best display quality, please access this system on computers.
Smart Connection System (i.e. check-in system)
When you step into a large conference, one hopes to interact with someone who shares a common interest. Common interests may include potential collaborations, supportive mentorships, the perfect job opportunity or even the right fit candidate for the job. These interactions or potential opportunities are often lost due to limited time interactions or transparency opportunities at a conference.
CABS has changed the missed opportunities to open opportunities with the newly launched CABS Smart Connection System. This allows you the opportunity to connect and interact with peers indefinitely. There are no more missed opportunities, but rather what are the new open opportunities.
Scan the QR code to connect with conference attendees and also you can obtain the electronic program book.

- Smart Connection System is a platform that CABS provides for BioPacific Conference attendees.
- On this platform, one can explore who attends the conference and make a connection to an attendee via email or phone number.
- The system also provides you with a place to publish your professional profile. You can select “Do NOT publish my contact info” if you do not want to disclose your contact info.
Online

Agenda:
• 5:30-7:00 PM Pacific - Social networking
• 7:00-7:05 PM Pacific - Brief opening (Kate/Carrie)
• 7:05-7:40 PM Pacific - Presentation by Helen Chen
• 7:40-8:40 PM Pacific - Panel discussion
• 8:40-9:00 PM Pacific - Q&A and networking
This is complimentary event, registration is required.
During online registration, please write in note section or email cabs_icc@cabsweb.org if you plan to attend in person. Free dinner and refreshment will be provided at the event.
Zoom link will be provided after registration.



International Conference Room, 755 Page Mill Rd, Palo Alto, CA 94304 (Morrison & Foerster);
The Chinese American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that Dr. SCOTT LIU is the winner of the 2022 CABS K. Fong Award in Life Sciences for his outstanding contribution to the development of multiple biologic products from research to launch. We will honor Dr. Scott Liu at an award ceremony during the 22nd CABS Annual Meeting of the BioPacific Conference, on Saturday, November 12, 2022 at the San Mateo Marriott San Francisco Airport Hotel (1770 South Amphlett Blvd., San Mateo, CA 94402).
Scott Liu, PhD
Scott Liu is the Founder, Chairman, and CEO of HanchorBio Inc., a global biotechnology company pioneering the development of next-generation immunotherapies. As a life-science entrepreneur and successful biotech company-builder, Dr. Liu was one of the global partners of Fosun International Limited and Co-founder, President, and CEO of Shanghai Henlius Biotech Inc., a commercial-stage global biopharmaceutical company with the focus on high-quality, affordable, and innovative biologic medicines, listed on Hong Kong Stock Exchange (2696.HK) with a market value of over US$ 1.1 Billion. During his tenure with Henlius, Dr. Liu led multiple product development initiatives (over 30 biosimilars, novel monoclonal antibodies, and bispecific antibodies) and successfully launched 5 commercial monoclonal antibody products in China and Europe.
Dr. Liu has over 30 years of experience in managing corporate development, strategic portfolio, equity, cGMP quality operation, and CMC regulatory affairs. He has been instrumental in the development of the Technical Guidelines for the Research, Development and Evaluation of Biosimilars to promote globalization of the biopharmaceutical industry in China. Additionally, he has participated in development of multiple biological medicines, including Orencia® (for rheumatoid arthritis), Vectibix® (for colorectal cancer), Hanlikang® (rituximab biosimilar), Hanquyou®/Zercepac® (trastuzumab biosimilar), Handayuan® (adalimumab biosimilar), Hanbeitai® (bevacizumab biosimilar) and Hansizhuang® (serplulimab, novel anti-PD1).
Prior to founding HanchorBio and Henlius, Scott has previously served several executive positions such Vice President of Scientific Affairs at United Biomedical Inc., the Founding Director of the Biologics Quality Control Department at Bristol-Myers Squibb (Syracuse, USA), and the Director of Quality Analytical Laboratories at Amgen (Fremont, USA). Dr. Liu has authored or co-authored multiple scientific papers and has been an invited speaker in conferences with topics ranging from Biologics Process Development, Biomanufacturing, Oncology Biologics and Immuno-Oncology. He received his Ph.D. in biology at Purdue University and completed his post-doctoral training at Stanford University.
About CABS K. Fong Award in Life Sciences
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and the biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria are based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the CABS community and promotion of international collaborations in life sciences.
About Dr. Kenneth Fong
Dr. Kenneth Fong has spent the last 32 years in the biotech industry after completing his academic pursuit in biomedical research.
He is best known for founding the biotech company, Clontech in 1984 which he built into one of the largest biomedical tool companies founded by an Asian American in the US (400 employees including 65 PhD scientists). Clontech was sold to Becton Dickinson in 1999 and Ken has continued his career as a Venture capitalist with Kenson Ventures that he founded. He has since cultivated more than 10 highly successful entrepreneurs, advising them and working with them on the growth of their companies.
Currently, he sits on the board of 4 biotech companies and he was intimately involved with the M/A and IPO of more than 10 companies that are worth more than $3 billion. These companies range from research tools, medical diagnostics and drug development. In almost all cases, Dr. Fong has been instrumental in providing strategies for sustainable growth, value creation and liquidity. Those successful entrepreneurs have moved on to assume leadership in other start-up and mid-sized companies, which in turn led to a new generation of entrepreneurs.
Ken has held a number of leadership positions over the years. He served as the President of the Society of Chinese Bioscientists in North America (2006-07) and President of the Bay Area Asian American Manufacturers' Association (AAMA, 1987). He was also a member of the Board of Trustees of the California State University System (2006-13). His philanthropic interests include scholarships to San Francisco State University, the Kenneth Fong-Hearst endowed scholarships to the CSU system and 40 student scholarships to Peking University. In 2006, he was involved with establishing the Fong Optometry and Medical library at UC Berkeley, and more recently an endowed professorship at Stanford University and a technology translation endowed fund at San Francisco State University.
Ken obtained his PhD from Indiana University and his BS from San Francisco State University.
Past recipients of CABS K. Fong Awards
2021:Dr. John O. Link, Vice President of Gilead Sciences and Dr. Xian-Ping Lu, Chairman, CEO of Shenzhen Chipscreen Biosciences Co. LTD, for their extraordinary achievements in research, entrepreneurship, and innovation.
2019: Dr. John V. Oyler, Chairman, Co-Found and CEO of BeiGene, for his entrepreneurship and business leadership to establish BeiGene as a world-class biopharmaceutical company.
2018: Dr. Yuling Luo, Founder, CEO and Chairman of Alamar Biosciences, and Dr. Guoliang Yu, Executive Chairman of Crown Bioscience, for their successful serial entrepreneurship in the life science business.
2017: Dr. Yinxiang Wang, Co-founder and CSO of Beta Pharma, for his role in leading development and commercialization of Conmana®, the first small molecule oncology drug specifically targeting cancer cells that was completely developed in China, and Dr. Edgar Engleman, for his pioneering research that was the basis of the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA.
2016: Dr. Gerald Chan, co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, of Stanford University, for his pioneering work in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life- saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth, Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.online
Chinese Bioscience Association is hosting our 24th Annual Conference titled Next-Generation Medicine: From Gene, Protein to Cell Therapy
About this event
Please join us as we host Dr. Gregg Semenza, 2019 Nobel Laureate in Physiology/Medicine and Dr. Joseph Wu, Director of Stanford Cardiovascular Institute as our keynote speakers. We have also invited other distinguished speakers in antibody therapeutics, CAR-T cell therapy, gene editing and regulatory affairs.
Early bird registration begins today and ends August 31st. Grab your tickets now! https://cbasf.org/
https://www.eventbrite.com/e/chinese-bioscience-association-annual-conference-2022-tickets-391481831697
Crowne Plaza Foster City-San Mateo 1221 Chess Drive Foster City, CA 94404
Free Online Workshop: Immunogenicity of Biologic Drugs - Clinical Relevance case studies, Predictive Tools & Challenges with New Modalities
Date: September 15th - 16th, 2022 @ 8:30 am - 12:30 pm (PST)
Venue: Online via Zoom by PBSS-SF Bay Area
Organization: PBSS
Details & Registration: https://www.pbss.org/aspx/eventInfo.aspx?eID=779
Online via Zoom by PBSS-SF Bay Area
ChinaBio® Partnering Forum 2022
Beijing and Digital
Sep 13-14, 2022 Beijing
Sep 15-16, 2022 Online
ChinaBio® Partnering Forum is the largest and most productive life science partnering event in China. This 4-day hybrid event will bring together biotech and pharma leaders from around the world along with hundreds of China-based developers of novel technologies for four days of successful partnering and feature a blend of live and on-demand program sessions, company presentations, table-top exhibits, company showcases, and networking sessions.
CABS subscribers will enjoy 20% registration discount off the regular rate using promo code ‘CABS20’.
北京和虚拟会议
2022年5月10-13日
ChinaBio合作论坛是中国最大最富成效的国际性生命科学合作会议,将于2022年9月13-14日通过线下现场和线上虚拟的组合方式举行。此次会议将汇聚来自世界各地的生物技术和制药业领袖以及数百名中国新技术研发企业,进行为期四天的成功合作互动。去年,会议吸引了来自 27 个国家 500 多家公司的 1,000 多名代表,使其成为有意在中国境内外发展跨境合作关系的公司必须参加的全球盛会。除一对一合作会议之外,本会议还包括现场和点播观看的会议演讲、公司路演、桌面展览、公司展示以及各种社交环节。
CABS会员凭优惠代码CABS20,享受原价基础上8折注册优惠!


hybrid (Beijing and Online)
Antibody-based drugs have revolutionized the treatment of many previously untreatable diseases, including solid tumors, hematological malignancies, and autoimmune diseases. Due to the complex nature of antibody-based drugs, there are unique development challenges associated with the Chemistry, Manufacturing, and Controls(CMC) of biologics. CABS and Aton will cohost a seminar titled -Antibody-based Drug Development, from R&D to Commercialization on August 27th, 2022 at 10:00 am – 1:00 pm (PT). Join us and learn about the CMC development process from Investigational New Drug (IND) to Biologics License Application (BLA/market approval) from industry-leading experts.
Agenda
10:00 to 10:05 AM Welcome Remarks from CABS. Yan Wang
10:05 to 10:35 AM CMC Considerations during Drug Development and Commercialization. Wei Huang
10:35 to 11:05 AM From IND to BLA, CMC Considerations from FDA’s Perspective. Sandy Yan
11:05 to 11:35 AM From DNA to IND & Beyond: Considerations for Biopharmas to Outsource CMC to CDMOs. M. Tim Tian
11:35 to 12:05 PM Enable A Novel Therapeutic Antibody Clinical Trial in 12 months. Fan Chen
12:05 to 1:00 PM Panel Discussion and Networking Lunch.
This event will be available in-person and via Zoom. Please confirm in-person in “Notes” during registration
Suite 280, 1633 Old Bayshore Hwy, Burlingame, CA 94010
Have you always wanted to improve public speaking skills but don't know where to start?
Would you want to get a taste for public speaking in a relaxed, outdoor setting?
You are invited to the open house hosted by the BioPacific Toastmasters Club on Sat, Aug 20th, 2022, 10:00 AM-12:30 PM PDT at San Mateo Central Park.
You will hear wonderful speeches while enjoying the blue sky and fresh air.
We will also celebrate our club 5 year anniversary. We invite all the past members or guests of BTPM to have a reunion party. You are welcomed to bring some snacks/food to share.
Please find more details and register at: https://forms.gle/VunfNE93sayskNnA6
.png)
San Mateo Central Park (50 E 5th Ave, San Mateo, CA 94401)
|
Free Online Symposium: Advanced in Cancer Therapeutics Date: August 17th - 18th, 2022 @ 8:30 am - 12:30 pm (PST) Venue: Online via Zoom by PBSS-SF Bay Area Organization: PBSS |
Online via Zoom by PBSS-SF Bay Area

Dear friends, do you want to develop your professional network and enhance your business profile?
Do you want to take part in shaping exciting events that for our life sciences community?
Do you want to hone your leadership and communication skills?
Do you want to make some friends and have fun?
Then come and volunteer for the Chinese-American Biopharmaceutical Society (CABS)! CABS is a non-profit and completely volunteer-based organization dedicated to serve the life science community. It is headquartered in San Francisco and has more than 3,000 members and 20,000 subscribers. Over the years, the CABS have organized numerous events for members of the life sciences community to share knowledge, build connections, and advance careers, and for biotech companies to establish partnership and collaborations.
There are many volunteer options at CABS: (1) You can volunteer at single or multiple CABS event(s) such as CABS annual BioPacific Conference, science and technology seminars, social gatherings, etc. (2) You can volunteer for different CABS committees to facilitate the fulfillment of their tasks.
As a volunteer of CABS, you will enjoy at least following benefits: (1) Ready access to a comprehensive and tightly knitted network of professionals in life science and the biopharmaceutical industry. (2) First hand insight into how professional organizations are operated. (3) Free access to the events you are helping with. (4) Volunteers with demonstrated capacity and commitment with be considered to become a member of the CABS Executive Committee. Executive members will enjoy additional benefits such as one-year free CABS membership (worth of $30) and up to two terms of membership fees for a toastmaster club (worth of $90)!
For more information and to apply, please click the link to our online registration form https://forms.gle/pbp6nfL11SycmZvm6
CABS Executive Committee

First impressions matter. According to a study conducted by the Harvard Business School, it takes just 7 seconds to form a solid impression on another human being. Over 50% of this is formed visually—observed through posture, appearance, dress choices, and the presence or absence of eye contact.
In today’s ever-demanding job climate, your external presence affects future opportunities, professional connections, and how effectively you are able to influence others. It is therefore imperative to observe, target, and enhance personal image to one’s fullest capabilities.
Recipient of the Jane Iredia award, Lilly Cook has been working in the beauty industry for more than 30 years, leading and training professional stylists as a style counselor. Lead by Cook, this workshop will provide tools to enhance and develop your personal image—with an emphasis on external ornamentation, international etiquette, and other manifestations of one’s personality.
1633 Old Bayshore Hwy #280
How to prepare tax return after Biden’s tax proposal didn’t really passed as law. So, could we still enjoy Trump’s lower tax rate from his 2017 tax reform of The Tax Cuts and Jobs Act (TCJA)? Our firm has developed a unique niche practice to handle these complicate scenario from this tax reform. Our firm’s managing partner Mr. Bin Zhai, CPA, CFP® from Zhai & Wang, LLP, will host a tax seminar and help clients to understand how to quickly and correctly finish their tax return.
Date: 06/11/2022, Saturday
Time: 2:00PM - 3:00PM
Location: Virtual Meeting
Topics Mr. Bin Zhai, CPA will cover are as following:
1. 2022 tax rate and tax bracket updated
2. 2022 Tax Forms and Stimulus Related Benefits
3. How much income can be exempted before tax kicked in: a deduction vs. a credit
4. Investment capital gain tax rate has its own tax brackets
5. Which areas are CA tax not conformed with Federal tax regulation
6. How to make sure you can get Qualified Business Income (QBI) for 20% deduction on rental and business income
7. Do you have to report foreign income, bank info and financial assets?
8. RSU, ESPP and stock option selling strategies.
9. Tax planning strategy under the current law
Topic: 2022 Tax Seminar by CABS
Time: Jun 11, 2022 02:00 PM Pacific Time (US and Canada)
Join Zoom Meeting
https://us06web.zoom.us/j/6145583019
Meeting ID: 614 558 3019
Passcode: CABS2021
One tap mobile
+16699006833,,6145583019# US (San Jose)
+13462487799,,6145583019# US (Houston)
Dial by your location
+1 669 900 6833 US (San Jose)
+1 346 248 7799 US (Houston)
+1 253 215 8782 US (Tacoma)
+1 301 715 8592 US (Washington DC)
+1 312 626 6799 US (Chicago)
+1 929 205 6099 US (New York)
Meeting ID: 614 558 3019
Find your local number: https://us06web.zoom.us/u/kexMYd6Rm7
Biography:

Bin Zhai, CPA, CFP®, MBA, and Partner: a twenty five-year practitioner helping dozens of small companies from startup to growth. Mr. Zhai specializes in tax planning and preparation in individual, trust and corporation as well as small business accounting, payroll and benefits. He also practices in financial planning, and wealth management for high net worth clients and business owners. He starts his career in public accounting firms in
Virtual
Dear everyone,
Greetings from the Chinese American Biopharmaceutical Society (CABS), Business and Career Development committee!
This year we are going to relaunch the Career Advisory Network (CAN) program and we would like to sincerely invite you to participate as a mentor or mentee. CAN aims to cultivate members to be the next generation of healthcare/life sciences professionals and leaders through mentoring, skill building, and networking. It is a 6-month program with 1:1, small group mentoring meetings, or large group networking sessions. Mentors and mentees are expected to meet once monthly during the months of May through Oct 2022 (in person or virtually).
Matching will be based on a variety of factors such as background, mutual interest, locations. We will officially launch the 2022 CAN program in April (Kick-off meeting date TBD). Here is a tentative schedule for this year’s program:
- Mentor/Mentee registration opens: Monday, 3/28/2022
- Registration closes: Monday, 4/18/2022
- Program acceptance, email notification sent: Friday, 4/29/2022
- Kick-off event: Early May
- Mentoring groups convene: May to October
- Graduation: Early December
Please visit https://www.cabsweb.org/can/ to learn more about the CAN program. If you are interested in becoming a mentor or mentee, please complete the forms below by midnight on Monday, 4/18/2022. - MENTOR registration:
- MENTEE application:
If you have any questions, please contact the chairs of the CAN Program Danielle Lee (danielle.lee2008@gmail.com)
or Jack Zhu (jackningzhu@gmail.com)
Best regards,
Danielle Lee and Jack Zhu
Chairs of CAN program
Online
The Chinese Bioscience Association, San Francisco (CBA SF) is delighted to invite you all to participate in our first activity in 2022. After enduring the hardships of the last two years of COVID-19, we have seen how Moderna and BioNT-Pfizer and Regeneron saved the world in 2020 by providing mRNA vaccines and antibody treatments to tackle the pandemic. However, investors are moving away from biotech stocks as COVID wanes. Today, their stock performance doesn't seem to reflect their success. In 2021, we faced a 25% collapse in 2021 and biotech stocks were down nearly 20% after the first month of 2022. One might ask “Do we expect to see this devastating fall turn around soon?” “How can startups raise their funds under this challenging environment?” “Which of the following is more intriguing to investors: the technology platform or innovative therapeutics?” “How easy is it to get the fundraising cross boarding under the current US-China climate?”
Our four speakers are experts who specialize in biotech, medical devices as well as cross-boarding investment. There will be some idea sharing and an exciting discussion. Please come to join us on Mar 26, 2022 at 3:30pm via Zoom. Registration is free.
Zoom



Oct 30, 2021 08:30AM-4:30PM US Pacific Time
Meeting ID: 997 6447 7339
Passcode: CABS2021


CABS is one of the largest non-profit biopharmaceutical professional organizations in San Francisco Bay Area. We are very pleased to host our 22nd annual conference virtually on Oct 30, 2021. This year's conference theme is “Passion for Better Medicine - Leading Biotechnology and Innovation”. We are expecting to attract more than 600 attendees globally, from the life science and Biopharmaceutical industries. The conference features 2 keynote speeches, 19 podium presentations and 2 panel discussions. The covered topics include: cutting-edge science and technology including gene therapy, cell therapy and next generation cancer immunotherapy; patent and legal considerations in life sciences; latest developments in biopharmaceutical policies and regulations; cross-border investment trend and M&A deals in the life sciences field during the COVID-19 pandemic.
Highlight of 2021 BioPacific Conference
- Targeted Cas9/gRNA delivery for potential in vivo genome editing
- New technology to develop drugs for previously undruggable targets
- Winners of the 2021 CABS K. Fong Award in Life Sciences
- Machine learning for drug development
- Cancer immunotherapy in the post checkpoint Inhibitor era
- New technologies in cell therapy
- A first-in-class small molecular drug developed for HIV treatment
- Regulatory considerations for early cancer detection and precision oncology
- Status review of COVID-19 pandemic
- Investment trend and deal discussions in biopharmaceutical industries
- IP and patent highlights in recent years

Click Here to Register on Zoom Conference
 Click Here to Know About Our Speakers and Panelists
Click Here to Know About Our Speakers and Panelists

Click Here to Know About Our Speakers and Panelists
|
Morning Session |
|
|
8:30 AM – 8:50 AM |
Breakout Room Networking |
|
8:50 AM – 8:55 AM |
Welcome Remarks Carrie Wang, MD, President-Elect of CABS and 2021 BioPacific Conference Organizing Committee Chair |
|
8:55 AM – 9:00 AM |
State of the Society Yang Tian, PhD, President of CABS |
|
9:00 AM – 9:35 AM |
Keynote 1: Traffic of Cas9-gRNA Between Cultured Human Cells Mediated by Cell-Cell Contact Randy Schekman, PhD, Professor, Department of Molecular and Cell Biology, UC Berkeley 2013 Nobel Prize for Physiology or Medicine |
|
9:35 AM – 10:00 AM |
CABS K. Fong Award in Life Sciences Fireside Chat, Presenter: Kenneth Fong, PhD, Chairman, Kenson Ventures LLC Awardees: John O. Link, PhD, Former VP, Gilead Sciences, Inc (Retired) Xian-Ping Lu, PhD, Founder, Chairman and CEO, ChipScreen BioSciences |
|
10:00 AM-10:25 AM |
K. Fong Award Speech: Reshaping Biotech Landscape in China Xian-Ping Lu, PhD, Founder, Chairman and CEO, ChipScreen BioSciences Co. |
|
10:25 AM – 10:50 AM |
K. Fong Award Speech: Lenacapavir: A Twice-Yearly Dosed First-in-Class HIV Capsid Inhibitor John O. Link, PhD, Former VP, Gilead Sciences, Inc (Retired) |
|
10:50 AM – 11:20 AM |
Cancer Immunotherapy Trends and Highlights in the Post-Checkpoint Inhibitor Approval Era Gary Starling, PhD, CSO, Xyphos Biosciences, Astellas Pharma Inc. |
|
11:20 AM – 12:00 PM |
Panel Discussion 1: IP Considerations and Investment Trends for Life Sciences Session will cover intellectual property considerations for life science transactions, investment stories of new technologies, and discussions on a new company structure. Moderator: Janet Xiao, JD, PhD, Partner, Head of China Life Sciences, Morrison & Foerster LLP Panelists: Chuck Comey, Partner, Morrison & Foerster LLP Sean Cao, MBA, PhD, Managing Director, CBC Group Neil Desai, PhD, Founder & CEO, Aadi Bioscience, Inc |
|
12:00 PM – 12:30 PM |
Breakout Room Presentations Room 0: How to Choose Key Reagents for CAR-T Cell Therapy Development Teng Peng, PhD, Technique Application Manager, ACROBiosystems Room 1: Effective Use of Science in the FDA Regulatory Process Bethany Hills, Partner, Morrison & Foerster LLP Keunbong (KB) Do, JD, PhD, Associate, Morrison & Foerster LLP Room 2. Precise and Efficient Non-viral CRISPR Gene Editing Solutions Lumeng Ye, PhD, Sr. Scientist, GenScript USA Inc. Room 3: Humanized Models for Improved Preclinical Discovery and Assessment of Novel Immunotherapies Jenna Frame, PhD, Manager, Scientific Communications & Marketing, Biocytogen Boston Corp. Room 4: Atomic-resolution cryoEM and Its Impact on SBDD Larry Jin, PhD, COO, Wuxi Biortus Biosciences Co., Ltd. What’s Ahead for the Biotech Industry: Another Wave or Low Tide? Michael Zhao, Co-founder, Q Bay (Boston) Room 5: Clinical Study in the US and China Charles Li, MBA, MS, VP, Business Development, CR Medicon, a Pharmaron Company Next-Generation DMSO-Free & Serum-Free Cryopreservation for Cell Therapy Manufacturing and Processing Xiaoxi Wei, PhD, Co-founder & CEO, X-Therma Inc. User Affiliate at Lawrence Berkeley National Laboratory Room 6: Future of Targeted Therapies in Lung Cancer Sanjeev Redkar, MBA, PhD, President and Co-Founder, Apollomics Inc. Room 7. Opportunities and Challenges in the Development of an Innovative Clinical Research Platform Sophia Kan, Director of Marketing Strategy, GoBroad Healthcare Group Alex J. Zhang, PhD, Chief Scientific Advisor, Hanhai International Inc. |
|
Afternoon Session |
|
|
12:30 PM – 1:05 PM |
Keynote 2: Towards Universal Druggability Greg Verdine, PhD, Professor, Chemical Biology, Harvard University |
|
1:05 PM – 1:35 PM |
How We Use Stem Cells & Genomics for Guiding Precision Medicine Joseph C. Wu, MD, PhD, Director of Stanford Cardiovascular Institute and the Simon H. Stertzer, MD, Professor of Medicine and Radiology, Stanford University |
|
1:35 PM – 2:05 PM |
Designing Better Molecules Faster and with Less Expense: Illustrations of the Transformative Impact of Physics-Based Modeling Combined with Machine Learning Matt Repasky, PhD, SVP, Life Sciences Products, Schrödinger Inc. |
|
2:05 PM – 2:35 PM |
The COVID-19 Pandemic: A Status Report Arthur Reingold, MD, Professor and Head of the Division of Epidemiology at the School of Public Health, UC Berkeley |
|
2:35 PM –3:15PM |
Panel Discussion 2: Strategic Partnerships & Financing Trends for the Biotech Industry Moderator: Cheni Kwok, CLP, PhD, Managing Partner & Founder, Linear Dreams LLC Panelists: Edward Harrington, MBA, CFO, Genentech Guo-Liang Yu, PhD, CEO, Apollomics Inc. Dewan Zeng, MBA, PhD, Head of Search and Evaluation, Business Development, BeiGene, Ltd. Cariad Chester, Partner, TCG Crossover “TCG X” |
|
3:15 PM – 3:45 PM |
FDA Experience and Regulatory Considerations on Companion Diagnostics and Cancer Early Detection Test Sharon Liang, MD, RAC, PhD, VP, Regulatory Affairs and Quality Affairs, Burning Rock Dx, LLC |
|
3:45 PM – 4:00 PM |
A World-Class Healthcare Technology Innovation Platform at Hong Kong Science Park Carrie Ling, PhD, Assistant Director of Business Development (InnoHK), Hong Kong Science and Technology Parks Corporation (HKSTP) |
|
4:00 PM – 4:05 PM |
Conference Adjourned |
|
4:05 PM – 4:30 PM |
Breakout Room Networking |

Click Here to Register on Zoom Conference

Download 2021 BioPacific Conference Brochure

Thanks to our Sponsors!


Virtual Zoom Conference
Free Online Seminar: Radio-labeled Mass Balance and Metabolite Profiling Studies: Fundamentals, Best Practices, Regulatory Requirements, Applications, and Case Studies
Date/Time: Oct. 14 and Oct 15, 13:00-17:00 Eastern Time
Venue: Online via Zoom by PBSS-Boston
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeBoston.aspx
Zoom
EPPIC Global Presents:
Title - Healthcare Start-Ups: Opportunities, Challenges and Trends
When - 18 September 2021, 9:00-10:30am PDT
Registration - Free for EPPIC Global Members
$20 for Non-Members
Link- https://eppicglobal.org/engage/calendar/#id=115&cid=1707&wid=1401&type=Cal

online

Zoom
Oligo-Therapeutics Seminar
Organized by CABS Science & Technology Committee
Oligonucleotides have potential to revolutionize medicine due to their ability to target previously “undruggable” disease genes. The Chinese American Bio-Pharmaceutics Society (CABS) will host an Oligo-Therapeutics seminar on September 11th, 2021 at 1:00-4:30pm (PDT). Brought together Oligo experts from Ionis, Eli Lilly, and Amgen, the seminar will delve into the pioneering development of oligo-therapeutics from chemistry and delivery, biology/pharmacology, to clinical translation. Join us to get exposed to ground-breaking oligonucleotide science and case studies and to network with oligo industrial professionals.
Date: September 11th 2021, Saturday, 1:00-4:30 PM (PDT)
Zoom: https://zoom.us/webinar/register/WN_h_M02eYFSEKTWjN1WEwU7A
Agenda:
1:00 to 1:05 PM PDT Welcome Remarks from CABS. Xiang Yi, PhD, Co-chair, CABS Science & Technology
1:05 to 1:50 PM PDT Targeted Delivery of Oligonucleotide Therapeutics. Punit Seth, PhD, VP of Medicinal Chemistry, Ionis Pharmaceutics, San Diego
Abstract: Synthetic therapeutic oligonucleotides (STO) represent the third bonafide platform for drug discovery in the pharmaceutical industry after small molecule and protein therapeutics. No less than thirteen STOs have been approved by regulatory agencies and over one hundred of them are in different stages of clinical trials. STOs hybridize to their target RNA or DNA in cells via Watson-Crick base pairing and modulate their function to exert pharmacological effects. This unique class of therapeutic agents has the potential to target genes and gene products that are considered undruggable by other therapeutic platforms. However, STOs must overcome several extracellular and intracellular obstacles to interact with their biological RNA targets inside cells. These obstacles include degradation by extracellular nucleases, scavenging by the reticuloendothelial system, filtration by the kidney, traversing the capillary endothelium to access the tissue interstitium, cell-surface receptor-mediated endocytic uptake, and escape from endolysosomal compartments to access the nuclear and/or cytoplasmic compartments where their targets reside. Recent advances in the delivery of STOs will be presented.
1:50 to 2:35 PM PDT Discovery and Development of Oligonucleotide Therapeutics: Past, Present, and Future. Gosia Gonciarz, PhD, SR. Director, Internal Platform Leader, New Therapeutic Modality, Eli Lilly and Company, Indianapolis
Abstract: Nucleic-acid based therapeutics are emerging new drug modalities beyond conventional small molecules, peptides and monoclonal antibodies, that offer new therapeutic avenues and solutions for unmet medical needs. Different classes of nucleic-acid therapies including antisense oligonucleotides, aptamers, small interfering RNAs, microRNAs, messenger RNA, viral vectors and CRISPR/Cas9 system are being evaluated in clinical trials or have already received regulatory approval. RNA-based therapeutics can act through different mechanisms ranging from activation to silencing and knockout of the target gene. The versatility of modes of action makes them popular therapeutic agents for various medical needs.
Unmodified RNA molecules are unstable, rapidly degraded and cleared from circulation. After years of dedicated research, major challenges associated with poor potency, stability and immune response activation have been overcome and led to discoveries of potent and durable RNA therapeutics. Antisense oligonucleotides (ASOs) and short interfering RNAs (siRNAs) are the most advanced and promising examples of this newer class of medicines. First wave of RNA therapies has been developed for treatment of rare indications such as spinal muscular atrophy, hereditary TTR-mediated amyloidosis and acute hepatic porphyria as exemplified by regulatory approvals of Spinraza, Onpattro and Givlaari respectively, building excitement and confidence in this new class of therapeutics. The next wave of RNA therapeutics might offer treatments for broader indications such as cardiovascular disease. Recent clinical readouts with inclisiran, an siRNA designed to inhibit the synthesis of the proprotein convertase subtilisin-kexin type 9 (PCSK9) to lower low-density lipoprotein cholesterol (LDL-C), suggest that RNA therapies could offer attractive therapeutic solutions to broad patient populations. As demonstrated in phase 3 clinical trial, a single subcutaneous injection of inclisiran can lower LDL-C level for 6 months. Long duration of action and infrequent administration of siRNA therapies is very attractive from various perspectives, including patient convenience, lower cost of goods and most importantly will significantly improve patient adherence to medication and as a result improve disease outcomes.
2:35 to 3:20 PM PDT siRNA Therapy in Metabolic Diseases. Jun Zhang, PhD, Principal Scientist, Amgen, South San Francisco
Abstract: Since discovery in 1998, RNA interference (RNAi) become a widely used research tool to probe the importance of individual genes by knocking down gene expression. Ten years later, synthetic small interfering RNAs (siRNAs) were discovered with great potential as therapeutics to extend its targets beyond what can be achieved with small molecules and biologic. Recent progress in clinics further suggest that siRNA drugs are safe, effective with extremely long duration of action up to six months, presented revolutionary impact on pharmaceutical industry. These advantages are beneficial to chronic metabolic conditions such as T2D, NASH, cardiovascular disease (CVD), chronic lung conditions, which in general require therapeutics met high standards of safety, effectiveness, and easy administration. A summary of synthetic siRNA-based drug design and progress in therapeutic development will be discussed, with a focus on opportunities and challenges to overcome metabolism related disorders and syndromes.
3:20 to 4:05 PM PDT Delivery of RNA Therapeutics: The Great Endosomal Escape! Steven Dowdy, PhD, Professor, Dept. of Cellular & Molecular Medicine, UCSD, San Diego
Abstract: RNA therapeutics have great potential to selectively treat human disease, especially cancer, pandemics and CNS disorders. Due to their large 8-14 kiloDalton size and 20-40 or more negative phosphate charges, RNA therapeutics are too large to diffuse across the cell membrane and are instead taken up endocytosis. However, >99% of RNA therapeutics remain trapped inside of endosomes with <1% escaping across the endosomal lipid bilayer membrane into the cytoplasm of cells. Consequently, endosomal escape remains The Technological Problem to solve for development and efficient delivery of all RNA therapeutics. Viruses also have an same endosomal escape problem, but have evolved mechanisms to solve it. We developed new chemistry to synthesize a universal endosomal escape domain (uEED) that biomimics the mechanism that enveloped viruses use to escape the endosome. uEEDs can be conjugated to all RNA therapeutics.
4:05 to 4:30 PM PDT Panel discussion
Speakers Background:

Dr. Punit P. Seth heads medicinal chemistry and targeted delivery at Ionis Pharmaceuticals. Dr. Seth is co-inventor of Ionis’ Gen 2.5 platform which employs high affinity nucleoside modifications to enhance the affinity, stability and potency of ASOs in the liver and in extra-hepatic tissues. He is also the co-inventor of Ionis’ LICA (ligand conjugated antisense oligonucleotides) platform which enhances ASO potency by targeted delivery to cells and tissues of interest. He has extensive experience with using medicinal chemistry strategies to modulate the activity, pharmacokinetic and toxicological properties of oligonucleotide drugs. Dr. Seth is listed as co-author/co-inventor on over 200 peer-reviewed publications, issued patents and patent applications. He has a Ph.D. in organic chemistry from The Ohio State University.

Malgorzata (Gosia) Gonciarz is a Senior Director in Lilly’s Novel Therapeutic Modalities organization, where she leads a diverse group of scientists to design and develop RNA based therapeutics and enable their delivery to specific tissues. Dr. Gonciarz received a M.S. in Biochemistry from the University of Wroclaw, Poland and a Ph.D. in Molecular and Cellular Biology from University of Gdansk, Poland. She then went on to post-doctoral work at University of Utah where she studied structure and function of cellular components. Dr. Gonciarz joined Lilly in 2009 and started her journey in Biotechnology Discovery Research organization where she has applied her expertise in protein engineering, structural biology, and biophysical characterization to advance peptide-, protein- and antibody-based therapeutics. Through her various scientific roles at Lilly, she led teams from early discovery through clinical proof-of-concepts advancing Lilly’s pipeline across breadth of therapeutic areas.



Dr. Steven F. Dowdy is a Professor of Cellular & Molecular Medicine at UCSD School of Medicine and a cancer biologist, specializing in the development and delivery of RNA therapeutics, and G1 cell cycle control in cancer. He received his Ph.D. in Molecular Genetics from UC Irvine with Prof. Eric Stanbridge working on tumor suppressor genes, and performed his postdoctoral fellowship with Prof. Bob Weinberg at the Whitehead Institute, MIT, working on the biochemistry of the Rb tumor suppressor gene. His lab is focused on the molecular details of delivery of RNA therapeutics across the endosomal lipid bilayer and the synthesis of endosomal escape domains to overcome this billion year old rate-limiting barrier. His lab was the first to synthesize bioreversible, charge neutralizing phosphotriester backbone RNAi prodrug triggers that increase metabolic stability, pharmacokinetics and enhance endosomal escape. Dr. Dowdy sits on five biotech Science Advisory Boards and is an elected member of the Oligonucleotide Therapeutics Society (OTS) Board of Directors (2015-present). Previously, Dr. Dowdy was a Howard Hughes Medical Institute Investigator (1994-2012) and an Assistant Professor at Washington University School of Medicine, St. Louis (1994-2001).

Zoom
The Chinese American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that Dr. John O. Link and Dr. Xian-Ping Lu are the winners of the 2021 CABS K. Fong Award in Life Sciences for their extraordinary achievements in research, entrepreneurship, and innovation.

Dr. John O. Link
Dr. John O. Link has 30 years of experience in discovering and developing drugs as a medicinal chemist and project leader. John received his PhD in Chemistry under the direction of EJ Corey at Harvard University working on CBS reduction chemistry and a novel synthesis of amino-acids, now named the Corey-Link Reaction. John elucidated the inhibition mechanism of the immunosuppressant drug CellCept® along with the enzymatic mechanism of its target, inosine monophosphate dehydrogenase at Syntex/Roche Palo Alto. At Celera John worked in the inflammation and antiviral areas with three compounds entering clinical trials. John was a Vice President of Medicinal Chemistry at Gilead Sciences and led the project teams that discovered the hepatitis C NS5A inhibitors ledipasvir and velpatasvir (Harvoni® Epclusa®) and continued as the project leader through Phase I clinical trials. The NS3/4a protease inhibitor drug voxilaprevir was discovered in his group (Vosevi®). John is co-inventor on the patents disclosing these three approved drugs. Under his leadership, Gilead moved three small molecule HCV inhibitors from discovery to FDA approval. Since their approval from 2014 to 2016, those HCV drugs have cured 1.5 million HCV patients globally by mid-2017. He is also a co-inventor and was a project leader on the first-in-class twice-yearly dosed Capsid inhibitor lenacapavir that is in multiple late-stage clinical trials for HIV treatment or prevention. John was awarded the American Chemical Society’s 2015 “Heroes of Chemistry Award” for his contributions to the discovery of Harvoni®, and in 2017 received the inaugural “Male Ally” award from the “Women at Gilead” employee resource group.

Dr. Xian-Ping Lu PhD
Dr. Xian-Ping Lu is Chairman, CEO of Shenzhen Chipscreen Biosciences Co. LTD. Dr. Lu founded Shenzhen Chipscreen Biosciences 20 years ago with a group of US-trained professionals. He built Chipscreen Biosciences into a leading Chinese drug discovery and development company focusing on innovative small molecular therapeutics. ChipScreen is probably the only truly innovative, vertically integrated biopharma company in China. Using active compounds as probes to characterize proteome functions, this novel approach allows small molecules to be systematically screened as new therapeutic targets, enhancing the ability to create target-specific chemical drugs. The chemical genomics approach led to the discovery of Chidamide, a highly selective histone deacetylase HDAC inhibitor. The approval of Chidamide also broke China’s reliance on foreign imports for innovative drugs. The out-licensing of its overseas patents brought Chipscreen large milestone payments and made Chidamide the first original Chinese drug authorized for use in the US and other countries. This sets a precedent for China to change from a drug imitator to a creator.
Previously Dr. Lu was Director of Research at Galderma R&D in Princeton until 2000, the year he became visiting professor at China’s State Key Laboratory for Biomembrane and Membrane Biotechnology in Tsinghua University. He also participated in founding Galderma Research Inc. and Maxia Pharmaceuticals in San Diego around 1994.
Dr. Lu came to the US in 1989 for postgraduate fellowship study at the Department of Pharmacology, University of California in San Diego, followed by research fellowship at La Jolla Cancer Research Foundation (Burnham Institute). He obtained his Ph.D. in Molecular Biology and M.S. in Biochemistry from Peking Union Medical College, Chinese Academy of Medical Sciences, and his B.S. degree in Biochemistry from Sichuan University.
With over 30 years of biomedical research and biotech/pharmaceutical experience in various therapeutic areas, Dr. Lu is a skilled leader of diverse groups in global operating settings. He has published more than 100 peer-reviewed papers in prestigious journals including Nature, Science and The Lancet. He is the lead inventor on over 300 patented inventions in areas of small molecule therapeutics.
In the past 20 years, Dr. Lu has participated and promoted many reforms in regulatory environment, policy, and guideline of NMPA, reimbursement system of national health care, security and exchange of capital market, taxation and many others in China. He has been recognized as “China’s New Drug Trailblazer” as well as winning various prizes and recognitions from the pharmaceutical industry and other institutions.
About CABS K. Fong Award in Life Sciences
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and the biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria are based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the CABS community and promotion of international collaborations in life sciences.
About Dr. Kenneth Fong
Dr. Kenneth Fong has spent the last 32 years in the biotech industry after completing his academic pursuit in biomedical research.
He is best known for founding the biotech company, Clontech in 1984 which he built into one of the largest biomedical tool companies founded by an Asian American in the US (400 employees including 65 PhD scientists). Clontech was sold to Becton Dickinson in 1999 and Ken has continued his career as a Venture capitalist with Kenson Ventures that he founded. He has since cultivated more than 10 highly successful entrepreneurs, advising them and working with them on the growth of their companies.
Currently, he sits on the board of 4 biotech companies and he was intimately involved with the M/A and IPO of more than 10 companies that are worth more than $3 billion. These companies range from research tools, medical diagnostics and drug development. In almost all cases, Dr. Fong has been instrumental in providing strategies for sustainable growth, value creation and liquidity. Those successful entrepreneurs have moved on to assume leadership in other start-up and mid-sized companies, which in turn led to a new generation of entrepreneurs.
Ken has held a number of leadership positions over the years. He served as the President of the Society of Chinese Bioscientists in North America (2006-07) and President of the Bay Area Asian American Manufacturers' Association (AAMA, 1987). He was also a member of the Board of Trustees of the California State University System (2006-13). His philanthropic interests include scholarships to San Francisco State University, the Kenneth Fong-Hearst endowed scholarships to the CSU system and 40 student scholarships to Peking University. In 2006, he was involved with establishing the Fong Optometry and Medical library at UC Berkeley, and more recently an endowed professorship at Stanford University and a technology translation endowed fund at San Francisco State University.
Ken obtained his PhD from Indiana University and his BS from San Francisco State University.
Past recipients of CABS K. Fong Awards
2019: Dr. John V. Oyler, Chairman, Co-Found and CEO of BeiGene, for his entrepreneurship and business leadership to establish BeiGene as a world-class biopharmaceutical company.
2018: Dr. Yuling Luo, Founder, CEO and Chairman of Alamar Biosciences, and Dr. Guoliang Yu, Executive Chairman of Crown Bioscience, for their successful serial entrepreneurship in the life science business.
2017: Dr. Yinxiang Wang, Co-founder and CSO of Beta Pharma, for his role in leading development and commercialization of Conmana®, the first small molecule oncology drug specifically targeting cancer cells that was completely developed in China, and Dr. Edgar Engleman, for his pioneering research that was the basis of the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA.
2016: Dr. Gerald Chan, co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, of Stanford University, for his pioneering work in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life- saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth, Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.online
17 Aug, 2021 | 05:30pm (PDT)
Online via Zoom | English
Join us and learn comprehensive knowledge from a group of top-notch researchers across the world how to effectively address emerging infectious diseases threats
Register Now: https://bit.ly/3xxKW0m

Zoom
Free Online Symposium: Advances in Immuno-Oncology
Date/Time: Aug 11 & 12, 2021; 8:30 - 13:00 (Pacific)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Zoom
To serve our community, the Chinese American Bio/Pharmaceutical Society (CABS) is delighted to host a FREE Virtual Job Fair on Zoom on August 7, 2021 from 2PM to 5PM Pacific time (5PM-8PM Eastern time). We have more than twenty entities that will participate in this event and intend to recruit talents for 80+ positions in US, Mainland China, and Hong Kong (as of July 15, 2021). See the flyer and registration link for job posting details. (Note that the job postings are continuously being updated on our website.) During the Job Fair, each participating entity will have an individual break room, where you will have opportunity to directly speak with the hiring team to explore career opportunities. There will also be a show room where featured entities will showcase their visions and talent recruiting objectives.
To register, please click Meeting Registration - Zoom, or scan the barcode on the flyer.
For entities that are interested in participating this event, we still have limited FREE spots available. You will have the privilege of 1) promoting job postings via CABS website prior to the event for free, and 2) an individual breakroom for your hiring team to directly interact with potential candidates on the day of the Job Fair. First come, first serve! Please contact cabs_bcd@cabsweb.org ASAP if interested.

Zoom
CBA SF is inviting you to a scheduled Zoom meeting.
Topic: Diet, Microbiota and Cancer by Dr. Pan
Time: Jul 31, 2021 10:00 AM Pacific Time (US and Canada)
Zoom
Free Online Symposium: Development of Inhalation Therapeutics
Date/Time: July 29 & 30, 2021; 8:30 - 12:30 (Pacific)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Zoom
CBA SF is inviting you to a scheduled Zoom meeting.
Topic: Checkpoint Inhibitors' Immune-Mediated Side Effects, A Perspective from A Non-Oncologist Physician.
Time: Jul 17, 2021 10:00 AM Pacific Time (US and Canada)
Join Zoom Meeting
https://zoom.us/j/94036197939?pwd=bm5sOFpGblRLelp2bVpIUDlyL3JxQT09
Meeting ID: 940 3619 7939
Passcode: 2021

Zoom
Free Online Seminar: Statins for Cholesterol Control: Historical Stories and Lessons Learned
Date/Time: July 15, 2021; 11:30 - 13:00 (Pacific)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Zoom
Gene editing based therapeutics have tremendous clinical potential and are currently being investigated for a variety of indications. However, several challenges need to be solved before the clinical potential of gene editing can be realized. In this presentation, Professor Niren Murthy (UC Berkeley) and Dr. Kunwoo Lee (CEO of GenEdit) will discuss the history of gene editing and present an overview of exciting recent developments in the field. Clinical stage ex vivo approaches targeting disorders like sickle cell disease, cancer, and HIV, and in vivo approaches targeting monogenic disorders in various tissues will be discussed. In addition, they will present recent work of their own, which is focused on solving the delivery challenges associated with in vivo gene editing. Following the presentation, Dr. Derek Sloan from GenEdit will moderate an audience Q&A session.

online
Have you always wanted to improve public speaking skills but don't know where to start?
Would you want to get a taste for public speaking in a relaxed, outdoor setting?
Or maybe you are just tired of Zoom meetings all the time and want to see some friendly faces in person?
You are invited to the open house hosted by the BioPacific Toastmasters Club on Sat, Jun 19th, 2021, 9:45-11:15 AM PDT at Ryder Park in San Mateo.
You will hear wonderful speeches while enjoying the blue sky and fresh air. Please find more details and register at:
https://forms.gle/imjE2M67x4KHgsYH8

Ryder Park: 1801 J Hart Clinton Dr, San Mateo, CA 94401
Free Online Symposium: Antibody-Drug Conjugates: Discovery to Development
Date/Time: May 26, 2021; 8:30 - 13:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
ChinaBio® Partnering Forum 2021
Shanghai and Digital
May 10-14, 2021
ChinaBio® Partnering Forum has grown to be the premier life science partnering conference in China since 2009. ChinaBio® Partnering Forum 2021, in its new hybrid delivery, will kick off with two days of productive in-person one-to-one meetings in Shanghai, China followed by three days of effortless virtual one-to-one meetings. That enables you to identify, meet and network with leaders across the life science value chain to accelerate your cross-border partnerships and expand your reach into the China market. ChinaBio® Partnering Forum 2020 was a busy three days, August 25–27, 2020, with 1,000 delegates representing 34 countries engaged in over 3,200 virtual partnering meetings. Additionally, delegates engaged with 8 program sessions, over 60 company presentations and over 20 Company Showcases, all available on demand and accessible from anywhere in the world.
ChinaBio® 合作论坛2021
上海和数字版
2021年5月10-14日
ChinaBio®合作论坛是中国首屈一指的生命科学合作会议。今年第十三届ChinaBio合作论坛组合版,将以崭新的线下和线上双重模式举行,会议前两天与会者在上海进行现场一对一合作会议、圆桌讨论和欢迎酒会等内容,世界各地的与会者则在会议后三天继续参与数字化的远程合作会议。这将帮助您找到生命科学行业价值链中的领先者,与他们会面并建立联系,从而加速您的跨境合作伙伴关系并进一步延伸您在中国市场的触角。去年首次数字版会议,凭借全球最大的生命科学合作活动提供商EBD Group提供的强大在线平台,来自34个国家/地区的1000多名与会者在为期3天的会议中进行了3200多次一对一会议、60个公司路演和20个公司展览展示。
CABS会员凭推广代码CABS20,享受20%的注册优惠。
Shanghai and Digital
Due to COVID-19 situation, CABS Board of Directors have decided to postpone CABS 2020 BioPacific Conference to October 30th, 2021 (Saturday). The conference venue will be the same: San Mateo Marriott Hotel. The election of 2020 CABS President-elect, Executive Council (EC) and transition of CABS presidency will also be postponed from September, 2020 to October, 2021. Current CABS President, President-elect and EC team will stay on duty until October, 2021.
CABS community's health and safety is our No.1 priority. We are one of the first professional organizations national-wide to cancel large social gathering events in anticipating the potential outbreak of Coronavirus infection back to January of this year. Postponing 2020 BioPacific Conference is a continuation of our policy to safeguard our community.
Current CABS EC team has been working together as a unified force to combat COVID-19 and serve our community. The team's willingness to serve for one more year is a testament of our strength and commitment to you during this crisis. CABS will continue leading our community efforts to combat COVID-19 and to provide more online forums to share the latest development in COVID-19 diagnostics and treatment and also in other areas of drug discovery.
For sponsors and exhibitors who have already committed to 2020 CABS BioPacific Conference, your reserved sponsorship/exhibitor spots will be carried over to 2021 conference. As an appreciation of your support at this critical moment, we will continue promoting your company brand names to our community from now until the end of 2021. There are still spots left for sponsor/exhibitors of 2021 BioPacific Conference. If interested, please contact fundraising@cabsweb.org for details.
A big thank-you to speakers/panelists who have already accepted our invitation to present at 2020 BioPacific Conference. We hope you are available for 2021 Conference on May 8th, 2021. CABS volunteers will reach out to you for details.
Finally, we sincerely thank 2019-2020 term CABS EC members and volunteers, for your team efforts in combating COVID-19 and your commitment to serve CABS for two years. Your efforts and diligent work will be special and important to our community.
Sincerely and Best
CABS Board of Directors and President
04/06/2020
Announcement
Scientific Seminar: Ongoing Battle Against Covid-19: Therapeutic Antibody Development
Time: April 24, Saturday, 4:30-7:00 PM Pacific Time; 7:30-10:00 PM Eastern Time; April 25, Sunday, 7:30 – 10:00 AM China Time)
Length: 2.5 hours
Format: Short Presentations and Panel Discussion
Webinar Platform: Zoom Conference (be able to host up to 1,000 attendees simultaneously)
Registration Link: https://bit.ly/3wCYpVv
Speakers Bios:
Henry Ji, Ph.D.
Chairman, President and CEO
•25+ years of experience in the biotechnology and life sciences industry
•Dr. Ji co-founded Sorrento and has served as a director since 2006, CEO and President since 2012, and Chairman since 2017
•During his tenure at Sorrento, he has engineered and led a phenomenal growth of Sorrento through acquisition and merger including Bioserv, ScilexPharmaceuticals, Concortis Biotherapeutics, LevenaBiopharma, LACEL, TNK Therapeutics, Virttu Biologics, Ark Animal Health, and Sofusa Lymphatic Delivery Systems
•Served as Sorrento’s Chief Scientific Officer from 2008 to 2012 and as its Interim CEO from 2011 to 2012
•Prior to Sorrento, he held senior executive positions at CombiMatrix, Strategen and also co-founded Stratagene Genomics, a subsidiary of Stratagene, and served as its President & CEO and Director of the Board
•B.S. and Ph.D.
Yang Liu, PhD
Chairman, Chief Executive Officer and Chief Scientific Officer, OncoC4, Inc. Rockville, Maryland
Chairman, AcroImmune, Guangzhou, China
Dr. Liu is currently the Chairman, CEO and CSO of OncoC4 and was the Director and Professor of the Institute of Human Virology and Department of Surgery at the University of Maryland at Baltimore. Dr. Liu has received many distinguished awards including the AAAS fellow. Dr. Liu’s laboratory established Sialoside-based pattern recognition as a mechanism of self-nonself discrimination in innate immunity. Recently, Dr. Liu co-founded and led OncoImmune, Inc to translate this original discovery into a drug for treatment of multiple human diseases. OncoImmunerecently obtained clinical proof-of-concept data in a multi-center, randomized double blind clinical phase III trial showing that our first-in-class new drug significantly accelerated clinical recovery and reduced disease progress of patients with severe and critical COVID-19.
Liang Schweizer, PhD
Chief Executive Officer and Chief Scientific Officer
Liang Schweizeris the Co-founder, President and CEO of HiFiBiOTherapeutics. Previously, she co-founded HarbourBiomed and served as its CSO, successfully transforming a technology platform company to an antibody drug discovery enterprise.
Before launching her entrepreneurial career, Liang served as Head of Asian Cancer Research at Sanofi, advancing Sanofi’s Asia-Pacific oncology pipeline as well as contributing to global oncology programs from preclinical to clinical efforts. Before joining Sanofi, Liang Schweizerwas a director at Bristol-Myers Squibb Company, heading a group of scientists at Leads Discovery and Optimization (LDO), overseeing 60+ in vitro pharmacology projects across different therapeutic areas. Under her leadership, the team made significant contributions to 4 marketed drugs and impacted over 20 clinical candidates. She is also a co-inventor of multiple BMS Immunoncology(IO) clinical candidates.
Liang Schweizergraduated from the University of Science and Technology of China (USTC) majoring in Biology. She received her Master’s degree in Microbial Engineering at the University of Minnesota with minor in Chemical Engineering. Liang earned her Ph.D. in Molecular Biology from the University in Zurich, Switzerland. Her postdoctoral training was with Dr. Harold Varmus, a Nobel Laureate, at Memorial Sloan Kettering Cancer Center (MSKCC), New York.
Bolyn Hubby, PhD
Chief Corporate Affairs Officer
VirBiotechnology, Inc.
Bolyn Hubby, Ph.D., has served as our Chief Corporate Affairs Officer since August 2020. In this role, she is leading our Public Private Partnerships (P3), Government Affairs, Corporate Communications, Patient Advocacy efforts, and is a member of Vir’s Research Leadership Team. Prior to this role, she served as our Senior Vice President of Research and P3 since June 2019 and served in other management roles at Vir since February 2018. From January 2017 to January 2018, Dr. Hubby was the Chief Scientific Officer at AgenovirCorporation, a gene editing company we acquired in May 2018. Prior to Agenovir, Dr. Hubby worked for Synthetic Genomics Inc. from 2013-2017, where she most recently served as Vice President of Vaccines and Antimicrobials. At Synthetic Genomics, Dr. Hubby and her colleagues advanced the company’s core synthetic biology technology for influenza vaccines into the clinic and developed novel RNA and antimicrobial platforms to address the rapid spread of infectious disease and the growing global threat of antibiotic resistance. Prior to Synthetic Genomics, Dr. Hubby served as Executive Director of Vaccines at LiquidiaTechnologies, Inc. and Head of Discovery Immunology at AlphaVax, Inc. Dr. Hubby received her B.A. in Anthropology from University of Georgia and her Ph.D. in Cell Biology from the University of Georgia, Department of Cellular Biology and Center for Tropical and Emerging Diseases.
Moderator
Dr. Michelle M Chen

Dr. Michelle Chen is Vice President and Head of Corporate Development at WuXi Biologics where she leads corporate strategies, M&A, licensing, strategic partnerships, joint ventures, newcoformation and other initiatives. As a biotech executive and entrepreneur, she has over 20 years of industry experience in drug discovery and development, diagnostics, and technology/product development, with a track record of success in partnerships, R&D, product development and launches. Prior to WuXiBiologics, she worked at global companies such as BioMarin, Merck, and Roche, as well as a number of biotech companies and startups in various functions.
Dr. Chen received her Ph.D. in Biochemistry from the University of Washington, did her postdoctoral work at UCSF, and received bioinformatics training at Stanford University. She is a board member of BayHelix and a life member of BayHelix, CABS and CAB.
Free Virtual Symposium
Stanford Drug Discovery Symposium 2021
Free Virtual Symposium
April 19 – April 20, 2021
The meeting has very high level speakers that include CEOs, CSOs, and Senior Vice Presidents from many pharmaceutical companies, government officials, and venture capital groups. This year’s virtual meeting will be open to all with no registration fee.
Details and registration: tinyurl.com/sdds2021

Free Virtual Symposium
Free Online Symposium: Challenges and Successes in Newly Approved Therapeutics
Date/Time: Apr 16, 2021; 8:30 - 12:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Title - The Story of Pfizer-BioNTech COVID-19 Vaccine".
Time - Sat, 3/27, 2:00-3:30PM PST
Where: https://lnkd.in/gwPq5_E (Click the link to register)
Would you like to hear a first-person account from the Director of Viral Vaccines at Pfizer, Dr. Kena Swanson, who works in Vaccine Research and Development and was closely involved in the discovery and development of Pfizer, one of the first vaccines approved by FDA in Dec'20 for treatment of CoV-2 infection? Come join us on 27th Mar'21 from 2:00PM-3:30PM PDT for a Q&A moderated by Dr. Colby Shemesh and Dr. Denison Kuruvilla!
online
您是否有过关于薪酬,晋升,或者职业规划方面的困惑? CABS非常荣幸得邀请到对北美生命科学行业人才培养和事业指导方面拥有丰富经验的Kay Tong女士 和Jonathan Wong先生于3月26日(周五)下午四点(美西时间)/七点(美东时间)做一个百分百真诚的线上分享 (具体信息见海报)。Kay和Jonathan希望此次活动能尽可能多的和参会者交流,因此这次活动将限于50名参会者。活动免费,无需注册,欢迎大家参加。Zoom link:https://zoom.us/j/99391671885,或扫描海报二维码。
(注:当参会者到达50人后,会议将被锁住,不再开放。所以请准时加入会议)。
另:如有问题希望Kay和Jonathan在panel中解答,请在3月19日之前email给youyuy@gmail.com。
Do you ever have questions about compensation/promotion/career strategies? Come to this CABS Happy Hour Zoom event on March 26 (Friday) 4PM Pacific (i.e., 7PM Eastern) to get an honest perspective of two insightful speakers, Kay Tong and Jonathan Wong. Kay and Jonathan have extensive experiences in career coaching and talent acquisition for entry and mid-level professionals in life sciences industry in the US. (See flyer for more information.)
This is a free event. No registration is required. As Kay and Jonathan prefer to have open discussion with attendees as much as possible, this zoom event will be limited to 50 attendees. Zoom link: https://zoom.us/j/99391671885.
[Note: the meeting will be locked after the number of attendees reach 50. Therefore, please join the meeting on time.]
If you have any questions that you hope Kay or Jonathan to address, please send the questions to youyuy@gmail.comby March 19.


Online Zoom conference (register on Zoom below)
Free Online Symposium: Cardiac Safety from Preclinical to the Clinic: Industry and Regulatory Trends in T-QT and Concentration-QT Studies for Assessing QT Prolongation Risks
Date/Time: Mar 23 & 24, 2021; 8:30 - 12:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Free Online Symposium: COVID-19 Pandemic - Rapid Pivoting from Oncological to Antiviral Small Drug Development
Date/Time: Mar 19, 2021; 10:30 - 11:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Free Online Workshop: Metabolite Identification: Strategies, Techniques and Case Studies
Date/Time: Mar 9 & 10, 2021; 8:30 - 12:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
COVID-19 Small Molecule Drug Discovery Webinar
Organized by CABS Science & Technology Committee
COVID-19, caused by SARS-CoV-2, is a global health and economic catastrophe. The main protease (Mpro) and the papain-like protease (PLPro) are essential for SARS-CoV-2 replication and thus are the compelling targets for small molecule anti-COVID-19 drug discovery. Regulating proteases in other viruses has been proven to be a successful pathway in the treatment of virus-caused diseases such as HIV and HCV. With progress on the understanding of these protein structures by collecting their high quality X-ray data, drug design and medicinal chemistry are being accelerated by computational tools to efficiently identify potent small molecule inhibitors. It’s of great hope that some breakthrough discovery may be achievable in a short period of time through integrated efforts and collaborations. We are happy to bring these scientists and specialists together to present you a CABS online webinar about COVID-19 small molecule drug discovery. You will hear their recent discovery and thoughts about how we should conquer this virus. You are welcome to join and we are looking forward to your participation and scientific discussion.
Date: February 27th 2021, Saturday, 1:30-5:30 PM (Pacific Time).
Zoom: https://zoom.us/j/97482889026
Agenda:
1:30 to 1:35 PM Welcome Remarks from CABS. Ken Zhang, PhD, Co-Chair, STC, CABS.
1:35 to 2:15 PM Drug Repurposing for Treatment of COVID-19 and Inflammation-related Diseases Guided by Novel Computational Algorithms. Chang-Guo Zhan, PhD, Endowed Professor of Pharmaceutical Sciences and Director of Molecular Modeling and Biopharmaceutical Center, College of Pharmacy, University of Kentucky.
Abstract: In this talk, I will briefly talk about the applications of our recently developed novel computational algorithms to small-molecule drug repurposing for treatment of COVID-19 and inflammation-related diseases. Particularly for COVID-19, we have identified drugs suitable for the antiviral treatment targeting SARS-CoV-2 main protease and anti-inflammatory treatment of the COVID-19 hyperinflammatory (or cytokine storm).
2:15 to 2:55 PM How to Stop the Heart of a Virus: Specific Inhibitors and Clinical Drug Repurposing to Target SARS-CoV-2 Main Protease using X-rays, Neutrons and Computation. Andrey Kovalevsky, PhD, Senior R&D Scientist, Neutron Scattering Division, Oak Ridge National Laboratory.
Abstract: Computer-assisted and structure-guided drug design strategies rely on atomic scale understanding of the target biomacromolecule traditionally derived from X-ray crystallographic data collected at cryogenic temperatures. Conventional protein X-ray crystallography studies are limited by cryo-artifacts and its inability to locate the functional hydrogen atoms crucial for understanding chemistry occurring in enzyme active sites. Neutrons are an ideal probe to observe the protonation states of ionizable amino acids at near-physiological temperature, such as the non-canonical Cys-His catalytic dyad of SARS-CoV-2 Mpro. Our X-ray crystal structures of Mpro collected at near-physiological temperatures brought rapid insights into the reactivity of the catalytic cysteine, malleability of the active site, and binding modes with clinical protease inhibitors. The neutron crystal structures of ligand-free and ligand-bound Mpro were determined allowing the direct observation of protonation states of a catalytic site in a cysteine protease for the first time. At rest, the catalytic Cys-His dyad exists in the reactive zwitterionic state, with charged thiolate and doubly protonated imidazole side chains, instead of the anticipated neutral state. Ligand binding results in modulation of the protonation states, retaining the overall electric charge of the active site cavity. Our research is providing real-time data for atomistic design and discovery of Mpro inhibitors to combat the COVID-19 pandemic and prepare for future threats from pathogenic coronaviruses.
2:55 to 3:35 PM Targeting the SARS-CoV-2 Main Protease for Drug Discovery. Wenshe Ray Liu, PhD, Gradipore Chair and Professor in Chemistry, Texas A&M University.
Abstract: By targeting the essential main protease of SARS-CoV-2, the COVID-19 pathogen, a series of small molecule antivirals have been developed and tested on their inhibition of SARS-CoV-2 infection of human cells. Potent inhibitors include bepridil, a FDA approved medicine and a number of aldehyde-based compounds that form a covalent bond with the catalytic cysteine in the enzyme active site.
3:35 to 3:40 PM Break.
3:40 to 4:20 PM Drug Discovery Targeting SARS-CoV-2 Main Protease and Papain-like Protease. Jun Wang, PhD, Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona.
Abstract: SARS-CoV-2 encodes two viral proteases, the main protease (Mpro) and the papain-like protease (PLPro). These two proteases cleave the viral polyproteins during the viral replication and are validated antiviral drug targets. In addition, PLpro has been shown to suppress host immune response. In this presentation, I will introduce our efforts in developing inhibitors targeting Mpro and PLpro. Through high-throughput screening and subsequent structure-based drug design, several inhibitors have been identified with both potent enzymatic inhibition and cellular antiviral activity against SARS-CoV-2. To elucidate their mechanism of action, we solved multiple X-ray co-crystal structures of Mpro and PLpro with inhibitors. The significance of our study is the discovery of dual inhibitors targeting both viral Mpro and host cathepsin L, both of which are critical for the viral replication.
4:20 to 5:00 PM Remdesivir’s Inhibition of SARS-CoV-2 RNA Polymerization. Jason Perry, PhD, Senior Director, Structural Chemistry Department, Gilead Sciences.
Abstract: (TBD).
5:00 to 5:30 PM Panel Discussion. Host and All Speakers.
Speakers Background:

Dr. Chang-Guo Zhan is an Endowed Professor of Pharmaceutical Sciences and Director of Molecular Modeling and Biopharmaceutical Center in the College of Pharmacy, University of Kentucky. He also serves as Director of Chemoinformatics and Drug Design Core of the Center for Pharmaceutical Research and Innovation (CPRI) at the University of Kentucky.
With a research activity ranging from basic sciences to practical clinical applications, Dr. Zhan has published more than 390 scientific papers in peer-reviewed journals and has 55 patents or patent applications. Dr. Zhan’s current research interests are mainly focused on rational design, discovery, and development of novel therapeutics, as well as drug repurposing. Two of designed novel therapeutic candidates designed by him have completed Phase II clinical trials in humans; one received the Breakthrough Therapy designation by the FDA and is currently in preparation of Phase III clinical trial. Another long-acting protein drug candidate is heading to Phase I clinical trial. He has been very successful in securing nationally competitive research funding from the NIH, NSF, DoD, and other funding resources, including more than $33M as PI since 2003.
At the University of Kentucky, Dr. Zhan was named as University Research Professor by the President and Board of Trusty in 2016, and won Kirwan Prize (the highest research award at the University of Kentucky) in 2017. He was a winner of 2005 Emerging Computational Technology Prize, American Chemical Society (ACS) Division of Computers in Chemistry, and is the current recipient of the NIDA Translational Avant-Garde Award from the NIH. Dr. Zhan was elected AAPS Fellow in 2010, and won 2016 AAPS Research Achievement Award in Drug Discovery and Development Interface.

Dr. Andrey Kovalevsky is a structural biologist, biochemist and neutron instrument scientist at Oak Ridge National Laboratory (ORNL). His current research focuses on the atomic level understanding of protein function, structure-guided drug design and drug resistance utilizing biophysical methods in combination with molecular simulations. Kovalevsky studied organic chemistry and crystallography at Kharkov State University, Kharkov, Ukraine, receiving his M.Sc. degree with Honors, and worked as a Research Assistant at Nesmeyanov Institute of Organoelement Compounds, Moscow, Russia. He received his Ph.D. in Physical Chemistry from University at Buffalo, The State University of New York. He studied macromolecular crystallography, biochemistry and molecular biology while working as a Postdoctoral Associate at Georgia State University. He held a Director’s Postdoctoral Fellowship and a Staff Scientist position while at Los Alamos National Laboratory. Currently, Dr. Kovalevsky is a Senior R&D scientist at the Neutron Scattering Division (ORNL).

Dr. Wenshe Ray Liu received his BS degree from Peking University in 2000 and PhD degree from UCDavis in 2005. He did two year postdoc in Scripps and started his independent research career at Texas A&M University in 2007 as an assistant professor. He was promoted to Associate Professor in 2013 and then Full Professor in 2016. Dr. Liu is the current holder of Gradipore Chair in Chemistry at Texas A&M University. Dr. Liu was a previous director of the TAMU Chemistry Mass Spectrometry Laboratory. He is currently chairing the Chemical Biology division in the Chemistry Department and the director of the Texas A&M Drug Discovery Laboratory. He is leading a chemical biology and drug discovery research group.

Dr. Jun Wang received the B.Sc in chemistry from Wuhan University in 2003, the M.Sc in chemistry from National University in Singapore in 2006, and Ph.D. in chemistry from University of Pennsylvania in 2010. He was a postdoc first at the University of Pennsylvania, then at UCSF. He started his independent career as an assistant professor at the University of Arizona, College of Pharmacy in 2014. He was promoted to associate professor with tenure in 2020. The Wang Laboratory focuses on developing antivirals targeting drug-resistant viruses and emerging viruses, including influenza A and B viruses, enterovirus D68 (EV-D68), EV-A71, coxsackievirus, poliovirus, and the recently emerged SARS-CoV-2.

Dr. Jason Perry is a Senior Director in the Structural Chemistry Department at Gilead Sciences. As a member of the modeling group for nearly 15 years, he has contributed to over two dozen research programs. His primary focus has been in the area of Antivirals, where he has helped in the discovery and characterization of five approved drugs. Prior to joining Gilead, he was an Applications Scientist at Schrodinger for 11 years, where he worked with researchers all over the US in getting the most out of their modeling software. He received his BA from Johns Hopkins and his PhD from Caltech, where he worked in the labs of Bill Goddard.
Online Zoom conference
Event Title: The “China Initiative” and Your Research Grants
Date: Thursday, February 25, 2021
Registration link: https://communications.dorsey.com/230/2356/landing-pages/rsvp--prefill.asp?sid=blankform
Zoom link will be provided after registration
Agenda and detailed event info (include any web links as applicable):
SCHEDULE & DETAILS
8:00 p.m. – 9:30 p.m. EST
5:00 p.m. – 6:30 p.m. PST
9:00 a.m. – 10:30 a.m. Beijing Time
.jpg)
online webinar
Biopacific Toastmaster is going to hold the evaluation contest and open house in one single meeting on Feb 20th 2021. Please join us for a session to learn from the evaluation contestants and to socialize!
Registration can be done through scanning the barcode in the flyer. Zoom link will be sent after registration is confirmed.

Online Zoom conference (register on Zoom below)
Free Online Symposium: Oxbryta® (Voxelotor) Tablets For Sickle cell Disease: The R&D Journey and Lessons Learned (a joint symposium by PBSS and CLSA)
Date/Time: Feb 16, 2021; 12:00 - 13:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Free Online Symposium: RNAi Therapeutics: Discovery, Preclinical and Clinical Development
Date/Time: Jan 19, 2021; 11:00 - 13:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Chinese American Biopharmaceutical Society (CABS) is proud to present 2021 Investor Forum at 4-7:30 pm PST on January 13, 2021, in conjunction with the JP Morgan Healthcare Conference.
The Investor Forum will be joined by 16 China- and US-based investors, business development executives, and entrepreneurs. The topics will focus on the trends, opportunities and challenges in capital investment, business development and M&A, next generation of innovative therapeutics. The impact of COVID-19 and US-China relationship on Biopharma investment will also be discussed. The Investor Forum will be from 4 pm to 7:40pm.
- 3 Panel Discussions; 16 High-Profile Guest Panelists
- Next Wave of Innovative Therapies
- Trends of Capital Raising in Biotech and Pharma
- Building Cross-border Life Science Companies
- Join at 4PM: Networking and Socializing over Zoom
- Stay Until the End: Meet with Panelists over Zoom

Free Registration https://zoom.us/meeting/register/tJwkd-qvrD4vGtMjSvn8jgY6Pomwme58UnND
Please Update to to Latest Version of Zoom (click here to update) before Join-in. Otherwise You Cannot Use Zoom Breakout Room Function.

Agenda (Based on US Pacific Time)
4-4:50 pm: Networking in Zoom Breakrooms
4:55-5:00 pm: Opening remarks by Yang Tian, CABS President
5-5:50 pm Investor Forum Panel 1: Invest in Next Generation of Innovative Therapeutics
Moderator: Janet Xiao, Partner, Morrison & Foerster LLP
Panelists: Jennifer Goldstein, Managing Partner, Silicon Valley Bank
Nick Naclerio, Founding Partner, Illumina Ventures
Vincent Xiang, Founder and Managing Partner, 7G Bioventure
Michael Salgaller, Supervisory Specialist, Invention Development and Marketing, NIH
Howard Edelman, CEO, SeeMedX
5:50-6:30 pm Investor Forum Panel 2: Trends of Capital Raising in Biotech and Pharma
Moderator: Alex Zhang, CEO, Hanhai Silicon Valley
Panelists: Cherry Lu, Founding and Managing Partner, RedHill Capital
Thomas Chou, Partner, Morrison & Foerster LLP
Michael Chan, Head of International Issuer Services, Hong Kong Stock Exchange
Helen Chen, Greater China Managing Partner, Head of China Life Sciences, L.E.K. Consulting
6:30-7:10 pm Investor Forum Panel 3: Building Cross-border Life Sciences Companies: Challenges and Opportunities
Moderator: Cheni Kwok, Managing Partner and Founder, Linear DreamsPanelists: Cynthia Cai, Venture Partner, Viva BioInnovator
Bin Li, Founder and CEO, Lake Bleu Capital
Hai Mi, Managing Partner, Panacea Venture
Scott Liu, Co-founder and Head of Strategic Advisory Committee, Henlius Biotech
7:10-7:40pm Meet with Panelists on Zoom
7:40pm End of event

Free Registration https://zoom.us/meeting/register/tJwkd-qvrD4vGtMjSvn8jgY6Pomwme58UnND

Investor Forum Panelists

Janet Xiao, PhD, JD, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Janet focuses her practice on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies. She advises her clients on patent matters relating to various technologies, including antibody therapeutics, cell therapeutics, nanomedicine, gene therapy, drug delivery systems, diagnostics, and nutraceuticals. Recognized by Chambers as being highly sought after for patent prosecution and strategy mandates by innovators from around the world, Janet develops and strengthens her clients’ complex patent portfolios to maximize their commercial value. She is instrumental in developing strategies for multibillion-dollar patent portfolios for pharmaceutical clients. Janet works extensively in performing IP due diligence reviews in the contexts of VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. She helps some of the world’s most-sophisticated biopharmaceutical companies and investors assess potential risks and devise preemptive risk-management strategies for their product development and investments. She has led hundreds of IP due diligence projects, and is noted for her in-depth knowledge of patent landscapes in the fields of cancer immunotherapy, gene editing, next-generation sequencing, liquid biopsy, antibody manufacturing, and medical devices. Janet works regularly with patent litigators in the event of legal challenges by third-party competitors or vice versa, and she is frequently called upon to represent clients in litigation and post-grant proceedings. She is among the very few IP attorneys in the world who are both equipped with solid skills in global strategic IP management and knowledgeable about IP issues unique to China and Chinese clients, and she has been the go-to counsel for IP issues in U.S./China cross-border transactions and operations in China. Since 2005, Janet has been actively involved in the leadership team of the Chinese-American Biopharmaceutical Society (CABS), a nonprofit organization dedicated to bridging the life sciences communities in the U.S. and China, including serving as president for the 2011–2012 term.
Jennifer Goldstein, Partner, Silicon Valley Bank
Jennifer Goldstein is the Managing Partner for Life Science & Healthcare at SVB Capital and a founding member of Venture Forward, a non-profit that provides resources and education towards developing a diverse, equitable, and inclusive venture ecosystem. She is also an advisory board member of Springboard Enterprises and the Women’s Health Innovation Coalition. A bioengineer by training, Jennifer is passionate about the role that venture investing can bring to discovery in the fields of health care and life sciences. She is currently leading SVB Capital’s Life Science & Healthcare investment team and helping develop the bank’s first life science direct investment fund for institutional Limited Partners. Jennifer has more than 20 years of experience in the venture and startup market segments, including leading and building several teams over the past 8 years at Silicon Valley Bank. Prior to that, she was a director on Pfizer's Venture Capital team, where she helped lead or co-lead investments into several life sciences companies, led fund-of-fund investment decisions, and independently managed Pfizer’s $250 million private equity portfolio. Jennifer has also served as a consultant on private equity deals across Europe while at Bain & Company and has held operational and research roles at Chiron, Genelabs, and Genencor. Jennifer graduated magna cum laude with a BSE in Bioengineering and a Master's of Biotechnology from the University of Pennsylvania. She holds an MBA from the Wharton School, where she was named a Joseph Wharton Fellow. She also serves as an independent director at Alexandria Real Estate Equities, Inc.
Nick Naclerio, PhD, Founding Partner, Illumina Ventures
Nick is the Founding Partner of Illumina Ventures. His passion is building companies at the intersection of technology and human health. Prior to joining the fund, he served as SVP, Corporate & Venture Development for Illumina where he was responsible for strategic planning, business development, licensing, venture investing and acquisitions. In six years, Nick and his team completed ten acquisitions, more than a dozen venture investments, and a multitude of other transactions including the formation of Helix and GRAIL and the creation of a global NIPT patent pool. Nick also served as the founding General Manager of Illumina’s Enterprise Informatics Business Unit. Before joining Illumina, Nick co-founded and was the Executive Chairman of the diagnostics company Quanterix. Previously he was Executive Chairman of True Materials and CEO of ParAllele BioScience, life science tools firms that were both acquired by Affymetrix. Nick got his start in venture investing at Motorola where he led the life science investment team and served on the boards of the SNP Consortium, Genometrix, Clinical Microsensors, and Orchid Bioscience. He founded and led Motorola Life Sciences, an early microarray company that was acquired by GE. Previously he held leadership positions at DARPA and served on the board of SEMATCH. Nick is chairman of Serimmune and Stilla Technologies, and also serves on the boards of Baebies, Cradle Genomics, DNA Script, Genome Medical, LetsGetChecked and Pattern Bioscience. Nick received a B.S. in Engineering from Duke University, was a Winston Churchill Scholar at Cambridge University, and earned a Ph.D. in Electrical Engineering from the University of Maryland.
Vincent Xiang, PhD, Founding and Managing Partner, 7G BioVentures
Dr. Xiang has worked in the life sciences industry for more than 20 years, with rich experience in scientific research, product development, company management, investment and mergers and acquisitions. He led the $550M acquisition of US Epic Pharma, the largest M&A ever done by a Chinese healthcare firm in 2016. Dr. Xiang has comprehensive practical experiences in leading and managing top fund investment and financing business for many years, and has a deep understanding of the capital market. The pursuit of excellence in clinical data and his extensive industry network make him a well-known investment expert in the field of international pharmaceutical investment and financing. From 2018 to 2020, Dr. Xiang was Head of BioVenture at Hillhouse Capital with responsibilities of global venture capital investment, team building, procedures determination, decision making for investment projects. He personally led 9 investment projects ranging from series A round to crossover round while his team invested 31 projects successfully. From 2016 to 2018, Dr. Xiang was the partner at 6 Dimensions Capital, which is a leading healthcare focused investment firm with an in- depth focus and extensive coverage across China and the United States. He led 8 projects and those 8 projects had been successfully set up in next round or already went IPO. He was awarded the best performance partner in investing achievements in 6 Dimensions. Prior to 2016, he worked as Managing Director and Head of international investments & business development at Humanwell Healthcare Group, Managing Director of Burrill Life Science Venture Capital Fund, Portfolio Manager/Analyst at Franklin Templeton. Dr. Xiang began his career at Sinopharm in China. Dr. Xiang received his Ph.D. in molecular biology from University at Stony Brook, MBA from the Wharton School, and B.S. in Immunology and Microbiology from Fudan University. He is a founding member of BayHelix Group, founders and board members of two life science startups.
Michael Salgaller, PhD, Supervisory Specialist of Invention Development and Marketing Unit, National Cancer Institute Technology Transfer Center
Dr. Michael Salgaller leads the Invention Development and Marketing Unit (IDMU) within the National Cancer Institute’s Technology Transfer Center, where he leverages over 20 years of business, scientific, and investment experience in various life science sectors to support licensing and technology development partnerships. The IDMU serves in a business development role to foster licensing and collaborative activity between buy-side stakeholders and the NIH. He is a long-time industry executive who held various positions in boutique professional service and early-stage biotechnology firms – as well as several years in government affairs and on the investment team of a venture capital firm dedicated to the life sciences. He is the author of “Biotechnology Entrepreneurship,” and teaches an entrepreneurship class at NIH. He is on the Board of Canines-N-Kids, a foundation supporting cooperation between researchers in pediatric and veterinary oncology. He has written over 100 articles, presentations, and book chapters. Dr. Salgaller received his PhD in Pathology from The Ohio State University.
Howard Edelman, Chairman of the Medical Device and Digital Health Screening Committee, Life Science Angels, CEO of SeedMedX
Howard Edelman is a medical device executive accomplished in all phases of medical product development and operations in a variety of organizational structures. Most recently, Mr. Edelman was General Manager of Cothera, a division of United Orthopedic Group, a global orthopedic company which provides medical devices and patient services into the secondary orthopedic market. Cothera was recently purchased by Breg in November of 2014. Previously, Howard Edelman was the Founder and CEO of VitalWear, a leader in thermal therapy. In 2009, VitalWear was named on the INC 500 list of fastest growing private companies and on the San Francisco Business Times Fast 100. In 2010, Medical Device & Diagnostic Industry listed VitalWear as one of the top 50 medical device companies to watch. Mr. Edelman has served in management roles in firms such as Johnson & Johnson, Zeiss, CooperVision, and Bio-Rad Laboratories and various startup companies in the Bay Area. Mr. Edelman is a member of the BayBio Expert Network and sat on the Research Committee for the Juvenile Diabetes Research Foundation. Mr. Edelman holds a B.S. in Biomedical Engineering from Boston University.
Alex Zhang, PhD, MBA, CEO of Hanhai Silicon Valley
Alex Zhang is the CEO of Hanhai Silicon Valley., an early stage investor and cross-border accelerator for startups in San Francisco Bay Area. Prior to Hanhai, Alex was the Managing Partner of Enverest, LLC., a Silicon Valley-based innovation solutions and investment advisory firm with branch offices in China and Singapore. Prior to co-founding Enverest, Dr. Zhang spent nearly five years at Thermo Fisher Scientific, where he was responsible for 4 business development deals exceeding $10 M, and played a key role in several billion-dollar acquisitions in MedTech. From 2001 to 2009, Dr. Zhang was a Senior Scientist at Tularik Inc. (acquired by Amgen in 2004), where he led drug discovery endeavors in oncology, cardiovascular and metabolic diseases therapeutic areas. Dr. Zhang received MBA degree at Cornell University, PhD in Organic and Analytical Chemistry at Texas A&M University, and BS in Chemistry at Shandong University. During his graduate research, he focused on the design and synthesis of therapeutic peptoids, as well as biological mass spectrometry. His research has led to publication of more than 20 peer reviewed articles and 4 patents. Dr. Zhang has served multiple leadership roles in the Executive Council of CABS, including as the President in 2017-18. Over the past decade, Dr. Zhang has advised a number of successful MedTech and digital health startups based in Silicon Valley.
Cherry Lu, Funding and Managing Partner, RedHill CapitalExperienced Partner with a demonstrated history of working in the leading venture capital and healthcare industry. Strong business development professional graduated from The Chinese University of Hong Kong and Tongji Medical Academy of Huazhong Science & Technology University. The details are as follow:– 13 years at Sequoia Capital China, from analyst to partner in Sequoia’s history – Led investment in 30+ MedTech/Biotech companies and many of them has become sub-segment leaders, e.g. Jafron (300529, a leading enterprise in China blood purification industry), Snibe (300832, IVD), Winner Medical (300888), Iray(688301), Armo (ARMO, Nasdaq), XtalpI(Industry pacesetter), etc. – Industry recognition: Forbes 2020 Top 25 Female Venture Capitalists in China; Forbes 2018 Top 25 Female Venture Capitalists in China; Instructor for Merck China Innovation Center; FMBA instructor at Cheung Kong Graduate School of Business
Thomas Chou, Partner, Morrison & Foerster
Thomas Chou is a co-head of the firm’s Asia private equity practice and a leader in the firm’s China M&A practice, both of which are consistently highly ranked in major independent industry league tables and lawyer ranking organizations. Thomas’ corporate practice spans over two decades of experience in the U.S. and Asia Pacific region, focusing on cross-border mergers and acquisitions (inbound and outbound), joint ventures, as well as private equity and venture capital financings. His clients include industry leading growth companies as well the investors who fund them (private equity and venture capital funds, family offices, corporate/strategic investment arms of MNCs, insurance companies, and sovereign wealth funds). His practice is focused on the healthcare and life sciences, technology, media and telecom, and consumer and retail industries. He is ranked as a leading individual in China for Corporate/M&A and Private Equity: Buyouts & Venture Capital Investment by Chambers Asia-Pacific 2020. He is also a leading individual for Private Equity and is highly recommended for Healthcare and Life Sciences by Legal 500 Asia-Pacific 2020. His clients pinpoint his “decades of venture capital experience both in the United States and in Asia,” with one noting: “He is patient, attentive to details and someone who has clearly conquered many battlefields.”
Michael Chan, Head of International Issuer Services, HKEX
Mr. Michael Chan, Head of International Issuer Services, leads the international IPO and issuer marketing efforts for HKEX. Mr. Chan is mainly focused on market development work promoting the Hong Kong listing platform and origination work attracting international and biotechnology companies to list in Hong Kong. In addition to leading the marketing efforts globally and working closely with prospects and constituents, Mr. Chan often speaks and serves as panelist and keynote speaker at major conferences and events locally and internationally. Prior to his current role, Mr. Chan has worked at the Listing Division of HKEX for 8 years covering areas of compliance, corporate governance, regulatory and IPO-related matters. Prior to joining HKEX, Mr. Chanhas worked at various financial institutions in corporate finance and business development. Michael has more than 18 years of experience in financial services and regulatory work.
Helen Chen, Greater China Managing Partner, Head of China Life Sciences, L.E.K. Consulting
Helen Chen is the Greater China managing partner of L.E.K. Consulting based in Shanghai, and a winner of the prestigious Global Leaders in Consulting for 2019 award from Consulting Magazine. She sat on L.E.K.’s Global Leadership Team, the firm’s governing board, from 2012 to 2016, and served as the head of L.E.K.’s China practice from 2011 to 2018 and Asia life sciences practice from 2015 to 2019. Helen has over 30 years of consulting and industry experience in the U.S. and Asia, and has resided in China since 2000. Helen is the head of L.E.K.’s China healthcare practice and a board member of L.E.K.’s Asia Pacific Life Sciences Centre of Excellence, supported by Singapore’s Economic Development Board. She has extensive case work and industry experience covering the full biopharmaceutical and medtech value chain, ranging from early research services to post-market product positioning and sales force effectiveness. In China, she has developed commercial strategies for international biopharmas and medtechs, investment thesis for financial investors, business plans for domestic startups and policy analyses for industry associations. Helen is a frequent speaker and author on the opportunities and issues in the China healthcare and life sciences, and has been quoted by publications including BioCentury, BioWorld, In Vivo, Wall Street Journal, South China Morning Post, Financial Times and Forbes Asia. Prior to joining L.E.K., Helen was an associate director of finance at Genentech (now Roche) and a sales planner at Abbott Laboratories (now AbbVie). She was on the Board of Pharmaceutical Management Sciences Association from 1995 to 1997. Helenreceived her A.B. cum laude in applied mathematics from Harvard University.
Cheni Kwok PhD, CLP, Managing Partner and Founder, Linear Dreams
Dr. Kwok is a senior biopharmaceutical executive with broad operational expertise who has executed over 150 transactions including M&A, strategic partnerships, licensing, divestitures, spin-offs and project financing. Dr. Kwok is the Managing Partner and Founder of Linear Dreams LLC, a management consultancy for the life sciences industry. The firm’s engagements include a broad range of business and corporate development activities including managing business development teams, product and technology licensing, search & evaluation of products and technology platforms, merger & acquisitions, corporate strategy, portfolio planning, market and competitive intelligence, due diligence support for financing as well as valuation services for 45 biopharmaceuticals companies, contract research & non-profit organizations, research institutes and investors in USA, Europe, China, Taiwan and Singapore. As Senior Vice President, Corporate Development at Poniard Pharmaceuticals Inc., Dr. Kwok established corporate and business development, strategic and commercial planning, new product planning, competitive intelligence and forecasting functions. Previously, she was Director of Business Development at Celera Genomics Inc., where she led the business development efforts for Celera's small molecule therapeutics, including the divestiture of the oncology pipeline (including Imbruvica® (ibrutinib)) to Pharmacyclics Inc. (now an AbbVie Company). Dr. Kwok held business development positions of increasing responsibility at Exelixis Inc., where she initiated multiple partnerships and served as the alliance manager for the GlaxoSmithKline PLC (GSK) collaboration. Prior to joining Exelixis Inc., she held various research management, technology assessment and alliance management roles at SmithKline Beecham PLC (now GSK). Dr. Kwok received a bachelor's degree with first class honors in biotechnology from the Imperial College of Science, Technology and Medicine, University of London, UK, a Ph.D. in human molecular genetics from the University of Cambridge, UK and has earned the Certified Licensing Professional (CLP) credential. At present, Dr. Kwok is serving as the Board of Directors of Chinese-American Biopharmaceutical Society (CABS) and serves on the Standards, Admissions & Recertification committee of Certified Licensing Professionals, Inc..
Bin Li, PhD, Founder and CEO, Lake Bleu Capital
Dr. Bin Li is the founder and CIO of Lake Bleu Capital, an Asian healthcare investment firm based in Hong Kong and Shanghai. Previously, he was a Partner at Ally Bridge Group and prior to that he was a Managing Director and the Head of Greater China Healthcare Research at Morgan Stanley. Since he moved to Morgan Stanley and established the healthcare research practice in Hong Kong in 2008, he had been ranked as the top analyst in China healthcare industry by the most widely recognized investor surveys, including: No. 1 Asia Healthcare Analyst by the Institutional Investor (II) for seven consecutive years (2008-2014); No.1 China Healthcare Analyst each year by II since the poll started in 2010 (2010- 2014); No. 1 in Wall Street Journal's “Best Asia Healthcare Analysts” in 2010 for his stock selection; No. 1 Asia Healthcare Analyst by other independent investor surveys including Greenwich and Asiamoney. Before joining Morgan Stanley, he was a senior healthcare research analyst at Merrill Lynch (Hong Kong) and responsible for the firm’s stock research efforts on Asia healthcare. Before moving to Hong Kong in 2007, he was a key member of a top-rank US pharmaceutical research team at Merrill Lynch based in New York. Dr. Li has over 20-year experience in healthcare industry. He received his Ph.D. in biochemistry and MBA from New York University and bachelor’s degree in chemistry from Fudan University, China. Prior to his sell-side career, he worked at Merck Research Laboratory as a scientist and published papers on peer-reviewed science journals.
Cynthia Cai, PhD, MBA, M. Eng, President, Tharton Consulting; Venture Partner, Viva BioInnovator; Senior Advisor, Northern Light Venture Capital
Dr. Cynthia Cai is an executive and investor with over twenty-five years of experience in healthcare and life science industry. International experience in sales, marketing, business development, and equity investment. In-depth understanding of global biotech and life science business, widely recognized as having a unique ability to bridge collaborations between technology and business, between the west and east culture. Currently, Dr. Cai is an investor targeting early-stage opportunities of innovation and disruptive technology in healthcare field. Prior to that, Dr. Cai had over 20 years of experience in leadership positions with one of the world’s most respected life science companies. As senior director of marketing in Agilent Technologies, she was responsible for its billion-dollar Chromatography, automation and Mass Spec. business. As business development manager and product marketing manager, Dr. Cai was involved in multiple acquisitions and divestitures, also led a $500+ million-dollar flagship product development and its global commercialization. Dr. Cai earned a B.A. and Master of Engineering from Tsinghua University, received her Ph.D. in Chemistry from the University of Massachusetts, and MBA from The Wharton Business School of the University of Pennsylvania. She served as Delaware governor economic advisor, a member of the Board of the Forum for Executive Women (FEW-DE). She has also been a mentor for Wharton EMBA, Tsinghua University Global MBA and Drexel LeBow School of Business for many years. Dr. Cai had been a regular speaker at healthcare conferences such as BIO; RESI; China Focus at J.P. Morgan, SAPA Annual Conference and Wharton China Business Forum.
Hai Mi, PhD, Managing Partner, Panacea Venture
Dr. Hai Mi is a Founding and Managing Partner of Panacea Venture. Prior to Panacea Venture, a life science VC focuses on early-stage investment and incubation, Hai joined Kleiner Perkins Caufield & Byers China as a Partner in 2015 and focuses on the firm’s life sciences practice. Prior to joining KPCB China, Hai was a partner at DT Capital responsible for investment in life science, healthcare and other related fields. Hai was a venture partner of SB China Venture Capital (SBCVC) and his investment transactions include BGI, SyMap Medical and Precise Light. Hai was Senior Director of Strategic Planning and then Vice President of Corporate Communications at WuXi Pharma Tech (NYSE: WX) in Shanghai where he played an important role in NYSE listing, and the acquisition of AppTec in US. He also served on the board of Chemspec International (NYSE: CPC) as an independent board director and the chairman of audit committee, and he participated in the privatization of Chemspec. Earlier Hai was a research scientist at Pfizer in the US. Hai obtained a PhD in Organic Chemistry from New York University, an MBA from The University of Chicago Booth School of Business, and a B.S. in Material Science from Fudan University in Shanghai.
Scott Liu, PhD, Co-founder and Head of Strategic Advisory Committee, Henlius Biotech
Dr. Scott Liu, the co-founder and Head of Strategic Advisory Committee at Shanghai Henlius Biotech Inc., a global biopharmaceutical company focusing on the development, production and commercialization of high-quality and affordable biosimilar, biobetter and novel therapeutic monoclonal antibodies. Dr. Liu has more than 25 years of experience in biopharmaceutical R&D, manufacturing and quality management. Prior to setting up Henlius in 2010, he has previously had postdoctoral training in Biology at Stanford University and has served several executive positions at United Biomedical, the Founding Director of the Biologics QC Department at Bristol-Myers Squibb Technical Operations, and the Director of QAL (QC) at Amgen Inc. Fremont (now Boehringer Ingelheim Fremont Inc.). Dr. Liu was the recipient of the Bristol-Myers Squibb “Technical Operations Presidential Award” in 2004. Furthermore, Dr. Liu led the promotion of industry-wide adoption of single-use manufacturing technology to support the production of clinical and commercial monoclonal antibody therapeutics, and actively participated in the development of Technical Guidelines of Biosimilar Development and Evaluation to promote the innovation and globalization of the biopharmaceutical industry in China. He received his Ph.D. degree in Biology from the Purdue University.

Free Registration https://zoom.us/meeting/register/tJwkd-qvrD4vGtMjSvn8jgY6Pomwme58UnND
Please Update to to Latest Version of Zoom (click here to update) before Join-in. Otherwise You Cannot Use Zoom Breakout Room Function.
Online Zoom conference (register on Zoom below)
Conference Name: 2021 CTIC 5 th Pre-JPM Healthcare Investment Summit
Date: January 9-10, 2021
Format: Virtual Conference
Conference Website: ctic-conferences.com
Host: CTIC Capital
Co-host: Bayhelix
Supporting Organizations: CABS, BioCentury, CLSI, CLSA
CTIC summit stands out as it was the only large conference during JPM where there were more investor attendees than company executives. We are also the only cross-border event with sponsorships coming from Asian venture investors for the past four years (check our previous summits).
2021 summit will feature live and on-demand presentations and discussions from CEOs, investors, and leaders in healthcare sectors:
Partnering Program: Investors to company executives ratio of 1:1
Investor Showcases: Featuring the US and Asian investors with their latest investment focus, personal stories, and insights
Company Showcases: Featuring selected global healthcare companies with cutting edge technologies (15 minutes presentation + 10 minutes on-screen discussion with a paired investor) Application submission deadline is December 26, 2020
Virtual Exhibition: Unlimited possibilities with the digital and interactive exhibition opportunity
Fireside Chats and Panel Discussion Topics: Latest updates and strategies for cross-border fundraising, business development, and collaborations
Virtual Networking Sessions
CABS member special invitation code: CABS2021 (20% off)
Registration Link: https://www.eventbrite.com/e/ctic-5th-pre-jpm-healthcare-investment-summit-tickets-128559391601
Scan the code to register

For more details about 2021 CTIC Healthcare Investment Summit, please visit ctic-conferences.com.
Virtual Conference
Free Online Symposium: Readying for the Vaccines: Latest Updates on COVID-19 Vaccine Development and Implementation
Date/Time: Dec 21, 2020; 11:00 - 13:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details & Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Free Online Symposium - Day 2: Preclinical Models to Guide Therapeutic Development in Oncology
Date/Time: Dec 15, 2020; 8:45 - 13:00 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
CABS Entrepreneurship Club Workshop
“Entrepreneurship and Investment“
Date:December 12, Saturday, 1:00-3:00 PM.
Location: https://zoom.us/j/99662926044
Zoom ID: 996 6292 6044

Speakers Bio:

Huijun Zhou, Ph.D., CABS Enterpreneurship Club Program Chair
Dr. Huijun Zhou is CEO and co-founder of Doctor Chain LTD and iDNA Inc. Dr. Zhou received her Ph.D. in molecular biology and genetics from Cornell University and completed her post-doctoral medical genetics training at the Stanford University School of Medicine. She is a clinical molecular geneticist, board-certified by the American Board of Medical Genetics. She currently serves as the Program Chair of Chinese American BioPharmaceutical Society (CABS) Entrepreneurship Club.

Hong I. Wan, Ph.D. is president, chief executive officer and director of Tallac Therapeutics. Previously as chief scientific officer and a member of the founding management at ALX Oncology, she led the team to advance ALX148, a best-in-class myeloid checkpoint inhibitor, for multiple tumor indications. Prior to ALX Oncology, Dr. Wan held various leadership positions at Pfizer and Wyeth Research, where she was responsible for the early development and translational medicine strategy and execution across various therapeutic areas. Dr. Wan started her career at Renovis where she led discovery programs in neuroinflammation. Dr. Wan received her Ph.D. in molecular and cell biology from University of California, Berkeley and A.B. degree in biochemical sciences from Harvard University.

Keting Chu is a scientist, entrepreneur and life science venture investor with a broad range of experiences in therapeutic development and venture investment.keting is Executive Chairman of the board at Bluejay Therapeutics currently. Keting was previously a partner and venture Partner of Lyfe Capital that is focused on healthcare VC investments in the US and China. Prior to joining Lyfe Capital, Dr. Chu was venture partner at Apple Tree Partners. Before joining Apple Tree Partners, Keting spent fiveyears as VP, Research TAP at The Leukemia and Lymphoma Society (LLS), and is responsible for venture philanthropy. Prior to LLS, Keting co-founded, as head of RnD or CEO, four biotech companies including Five Prime Therapeutics, Bio3, DigitAB and Mission therapeutics. Before that, Keting was the Head of Immunotherapy and Antibody Therapeutics Division at Chiron Corporation and she was responsible for the R&D pipeline of biologic. Keting received her MD in Sun Yat-sen Medical University specialized in infectious diseases in China, Ph.D. in Microbiology and Immunology at University of California at San Francisco (UCSF).

Lue Sun is now the Vice President of 3E Bioventures, a venture capital focus on healthcare investment. Prior to 3E, Lue worked at Fusion Fund in Palo Alto and led several investments, including Proscia, Loop Genomics, Quantapore, Huma.ai, and Subtle Medical. He received a Ph.D. in Biology from Tsinghua University before joined Fusion. By focusing on cross-sector innovation, Lue has invested and become the board member of Chigene and Subtle Medical since joining 3E. Meanwhile, he also covers deals in AI applications, Med Tech, Diagnostics, and Liver Diseases.
Please Mark Your Calendar
Annual CABS Entrepreneurship Club Innovation Roadshow | January 13, 2021 | Zoom
CALL FOR PROPOSALS
Online Webinar through Zoom Conference
Free Online Symposium: Engineering NK Cells for Immunotherapy
Date/Time: Dec 10, 2020; 12:00 - 13:30 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
Free Online Symposium - Day 1: Preclinical Models to Guide Therapeutic Development in Oncology
Date/Time: Dec 8, 2020; 8:45 - 13:00 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay

Time: December 7, 2020 (Monday) - 8pm ET, 5pm PST, US time
Registration: https://zoom.us/meeting/register/tJwvf--rqjsjEtFPbmlB0m3SvXCu59f9BHqS
Join us for the presentation, "From Foundations to Frontiers", a landmark study of the enduring contributions of Chinese Americans to American society from the 1800s to today.
The evidence-driven study details the contribution across the following seven industries: Arts&Culture, Civil Rights, Public Service and Politics, Business Leadership & Entrepreneurship, Infrastructure, Military & National Security, Public Health, and Science & Technology.
From Chih-Tang Sah and the semiconductor revolution to Chien-Shiung Wu and the Manhattan Project, Chinese Americans have played an integral, but often overlooked part in the technological growth of the United States.
Presented by Committee of 100 President Zhengyu Huang, this virtual presentation will provoke curiosity and offer a new perspective on Chinese American contributions, while identifying, quantifying and showcasing the contributions of the Chinese American community through data and storytelling.
The event will be a 30 minute presentation followed by a facilitated discussion.
Time: December 7, 2020 (Monday) - 8pm ET, 5pm PST, US time
Registration: https://zoom.us/meeting/register/tJwvf--rqjsjEtFPbmlB0m3SvXCu59f9BHqS
Speaker

Zhengyu Huang, President, Committee of 100
Zheng was born in Shanghai, China and came to the US at the age of ten. Zheng was educated at Stanford University, with a B.S. in Industrial Engineering, B.A. in Economics, and M.S. in Computer Science. He also earned his MBA from Harvard Business School. After graduation, Zheng worked at Intel Corporation and rose to Managing Director and then served under President Obama as a White House Fellow and special assistant to the Administrator for US Agency for International Aid. Thereafter, he started a financial service data firm that grew to 300 staff and 7 offices. He was most recently the Chairman of an education focused investment firm. He is the author of three books published in China that sold over 250,000 copies. He has lived in five countries, including the US, Germany, Japan, India, and China and traveled to over 80 countries.
Zoom

Free Registration at:https://zoom.us/webinar/register/WN_j4VcFTRTTSat9j-yCGYEZw
Speakers Bio

Janet Xiao, PhD
Patent Partner, Morrison & Foester LLPDr. Janet Xiao, patent partner at Morrison & Foerster LLP and head of firm’s China Life Sciences Group, focuses her practice on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies. Janet works extensively in performing IP due diligence reviews in the contexts of VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. Full bio

Yan Qi, PhD
Patent Associate, Morrison & Foerster LLPDr. Yan Qi, patent associate at Morrison & Foerster LLP, is committed to delivering targeted solutions to clients’ diverse intellectual property needs in the life sciences. Her practice includes patent preparation and prosecution, freedom-to-operate, landscape and patentability analyses, intellectual property due diligence, and patent litigation support. Yan applies her bilingual language skills to assisting crossborder matters involving Chinese companies. Full bio

Yuying (Kate) You, PhD
Patent Associate, Morrison & Foester LLPDr. Yuying (Kate) You, patent associate at Morrison & Foerster LLP, focuses her practice on patent preparation and prosecution, strategic portfolio management and IP due diligence. She helps emerging and established companies build strong patent portfolios in diverse technologies including biologics (such as antibody therapy), cell therapy, immuneoncology therapy, gene therapy, drug delivery systems and diagnostics. Full bio
If you have any particular question that you wish the speakers to discuss during the workshop, please feel free to send an email to Yuying (Kate) You via yyou@mofo.com.
Online Webinar through Zoom Conference
Free Online Symposium: The Drug Discovery and Development Journey of Polivy (Polatuzumab Vedotin): An Antibody- Drug Conjugate for the Treatment of Diffuse Large B-Cell Lymphoma
Date/Time: Nov 13, 2020; 11:00 - 13:00 (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
When - 31st October'2020 at 3:00pm - 4:30pm PDT
Register here - https://bit.ly/31lnJB8



Online
Free Online Symposium: No More Undruggables: Unconventional Approaches for Drug Discovery and Design - Prodrugs, PROTACS, Fragment-based Design, Directed Evolution and Beyond
Date/Time: Oct 29, 2020; 4:00 PM - 7:00 PM & Oct 30, 2020; 9:00 AM -12:00 PM (PST)
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online via Zoom by PBSS-SF Bay
.jpg)
.jpg)
We invite your participation to this exciting event by EPPIC Global, sponsored by Jubilant Biosys Limited on the role of AI/ML in drug discovery.
Machine learning, artificial intelligence, deep learning are buzz words we've all heard, as these technologies promise to transform the landscape of drug discovery. Hear about decoding biology through target-agnostic and engineering mindset approach. Learn about the power of linking different computational technologies needed for Lead Optimization such as multi-parameter optimization platform and experimental integration. Understand insights on pre-competitive consortia and public-private partnerships, different AI/ML approaches taken and expected output.
But how exactly do they work? What are the tools and various approaches? How AI is addressing the various challenges of the drug discovery process? What are the limitations of each approach? What it can/cannot do? What are the requirements for their successful implementation?
This session will be moderated by Anjali Pandey and will have presentations by Sharath Hegde, Stacie Calad-Thomson, Andrew Radin and Tarangini (Gini) Deshpande.
Title: Role of AI/ML in transforming and accelerating the drug discovery process
Timing: 17 October 2020, 3:00 PM- 4:30 PM, PDT
Register here for $10 - https://bit.ly/30EeD22
Online
We at the BioPacific Toastmasters club would like to invite you to join our first-ever virtual open house!
The theme of this special edition of open house is "Take Me Home, Country Roads". Everyone is encouraged to set your Zoom virtual background to something related to your hometown OR some objects you have that are related to your hometown, and everyone will have a chance to talk about it in the table topics session.
The open house will be held on Saturday, September 26th, from 6:00 to 7:15 PM. It will feature a prepared speech, an extended table topics session, and social time for the guests and members to get to know about each other as well as the club. The Zoom meeting link will be provided to the registered guests.
Please check out the attached flyer for more information. The registration link is https://forms.gle/bz1kUzAfv6ncB1xG9, which is also available in the flyer as a QR code.
New to Toastmasters? Check out https://www.toastmasters.org/membership/why-toastmasters for a great introduction.
Interested in our club? Visit https://biopacific.toastmastersclubs.org/

Zoom Conference

CABS Webinar
Leverage Global Clinical Resources for Drug Development
US Pacific Time: September 25 (Friday), 2020, 2:00pm-4:00pm

•How to Perform Early Stage Trials in US
•China Drug Regulatory Reform and Include China in Next Trial
•Manage Global Clinical Trials During COVID-19 Pandemic

Webinar is Free and No Registration Required
Join Webinar through Zoom:

Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
2:00pm - 2:05pm Introduction to CABS, Dr. Yang Tian, President of CABS
2:05pm - 2:40pm Phase I Clinical Trials in the USA – Maximizing Value, Managing Risk. Lisa E. Diamond, PhD, President, Frontage Clinical Services.
2:40pm - 3:15pm Including China into Your Drug Development Strategy: Why, When, and How. Jiansong Yang, PhD, CSO and SVP, Tigermed
3:15pm - 3:50pm China’s significant role in the handling of COVID-19 global pandemic. Melika Davis, MS, VP, and Le Dao, BS, PMP, Associate Director, BeiGene
3:50pm - 4:10pm Panel Discussion

Speaker

Lisa E. Diamond, PhD, President, Frontage Clinical Services
As President of Frontage Clinical Services since 2018, Dr. Diamond provides oversight for Clinical Study Conduct and Analyses, Client Relations, and Business Development activities.
Dr. Diamond joined Frontage as Director of Clinical Affairs in 2005, was Vice-President of Clinical Affairs and Project Management in 2009-2013, and rejoined Frontage in 2017 as Vice-President of Clinical Affairs, US and China. In these positions she managed cross-functional teams, including Data Management, Programming, Biostatistics, Project Management and Medical Writing, provided Clinical Operations oversight and supported Business Development and Client Relations. Her prior positions in academia, small biopharmaceutical companies, Palatin Technologies, DNX and Nextran (1989-2005) and at Bristol-Myers Squibb (2013-2017), contribute to a broad experience base in research and development, preclinical and clinical research, a detailed knowledge of GCP/ICH guidelines, and a solid background in oncology, immunology, diabetes, autoimmune disease and transplantation research.
Dr. Diamond earned her Doctor of Philosophy and Master of Science degrees in Basic Medical Sciences from New York University (New York, Ny), and her Bachelor of Science degree from Suffolk University (Boston, MA). She completed her post-doctoral training at Princeton University (Princeton, NJ), and is an adjunct professor at Rider University (Lawrenceville, NJ).

Jiansong Yang, PhD, CSO&SVP, Tigermed
Dr. Jiansong Yang is the Chief Scientific Officer of Tigermed, which is the largest clinical CRO in China. He’s also the Founder and CEO of Mosim (now a Tigermed company), where he leads a team of over 100 scientists focusing on drug development strategy, clinical study design and PK/PD data analysis.
Dr Yang has over 15 years of experience in the pharmaceutical industry, specialized in early drug development and clinical study design. Prior to Mosim, he was Director of Clinical Pharmacology at GSK. He received his Ph.D. on Clinical Pharmacokinetics from the University of Sheffield, UK.

Melika Davis, MS, Vice President,BeiGene
Melika is the VP of Global Clinical Operations at BeiGene and leads a team of about 400 associates around the world, responsible for the planning and execution of all Clinical Trials’ activities.
Melika has 15 years of senior management experience and 25 years of Clinical Research and Development Quality Assurance experience. Through designing and building successful global organizations, Melika and her teams have brought, on the worldwide market, 20+ products in the last 10 years, mainly in Oncology, Vaccines, and Anti-Infectives. Melika has led teams up to 700+ associates in Clinical Monitoring Operations and managed a portfolio up to 1,500 clinical trials a year.
Prior to joining BeiGene, Melika held numerous positions at Novartis and Schering Plough Research Institute of increasing responsibilities across the Research and the Development organizations in the areas of Oncology, Transplant, Anti-Infective, Respiratory, and Vaccines.
Melika holds a Master’s degree in Molecular Biology from Texas Women’s University in Dallas, Texas and a License de Physiologie from the University of Sciences in Nice, France.

Le Dao, BS, PMP, Associate Director, BeiGene
Le is an Associate Director of Portfolio and Program Management at BeiGene and is responsible for management of clinical programs in early stage development. She has 20+ years of clinical development experience and PM expertise that includes team management, business insights, clinical pharmacology (PK/PD) and combination product development across multiple disease areas, including oncology/CIT, immunology, and infectious disease. She has a strong record of collaboration across multidisciplinary functions in building and delivering on strategic plans. In addition to her technical industry expertise, she is passionate about making a meaningful difference in patients’ lives, community building, and leadership development.
Le holds a Bachelor’s degree in Chemistry from University of California, Los Angeles.

Webinar is Free and No Registration Required
Join Webinar through Zoom:
(Wechat user, please click upper right corner and choose "open in Safari", then you can add to calendar with one click)

Zoom Conference
Free Online Symposium: Regulated Large Molecule Bioanalysis: Fundamentals, the New FDA Guidance and Beyond
Date/Time: Sept 17 & 18, 2020; 8:30 - 12:00
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Online Webinar through Zoom Conference
Antibody Drug Conjugates Seminar
On August 29, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar on Antibody Drug Conjugates (ADCs).
With the accelerated approval of Trodelvy in 2020, nine antibody drug conjugates now on the market have truly proven their potential. During the webinar, five experts from Seattle Genetics, Sutro Biopharma, Genentech, and Eli Lilly delved into the pioneering development of ADC from its early discovery to manufacturing CMC and clinical study. The speakers also emphasized the recent application of the ADC in fields other than oncology.
The webinar attracted more than 200 attendees from the biotech and pharmaceutical communities.
Dr. Ken Zhang, Co-Chair of Science and Technology Committee of CABS, kicked off the webinar with an introduction of CABS and Science Committee’s previous events.
Dr. Kristin Bedard, Vice President of Immuno-biology of Sutro Biopharma, started with the first talk on “Antibody Drug Conjugates - Using a Novel Technology to Produce First in Class Immune Modulating Cancer Drugs”. Dr. Bedard introduced Sutro’s proprietary technology on ADCs production in a cell free environment for rapid screening of lead candidates. She showed lead ADC molecule STRO-002 with its in vitro and in vivo activity and strong synergism with checkpoint blockades such as PD-L1. “One advantage of STRO-002 is its dual functions; it kills tumor cells through immunogenic cell death and releases tumor antigens that can further stimulate anti-tumor immunity”. The second story Dr. Bedard shared was about ADC development combining of a novel toll-like receptor (TLR) agonist and a traditional cytotoxic antibody drug conjugate warhead. It demonstrated more complete anti-tumor effects in preclinical tumor models and protective T cell mediated immunity. “This novel ADC technology can not only reduce tumor burden through direct killing but can also stimulate a protective and adaptive immune response against the tumor”, Dr. Bedard concluded her talk.
Dr. Chunze Li, Director/Principal Scientist/Group Leader at Genentech, presented the second talk on “Clinical Pharmacology Strategies in Supporting Drug Development and Approval of Antibody Drug Conjugates”. Dr. Li started her talk with an overview of ADC design concept, i.e., to increase the therapeutic window of cytotoxic agents. She highlighted a few major challenges in ADC design. Dr. Li presented an overview of clinical pharmacology strategy to support the development and approval of an ADC. Specifically, she provided a few examples to illustrate the unique clinical pharmacology consideration for ADCs, including bioanalytical strategies, PK and ADME characterizations, exposure-response analysis to inform dose selection and strategy to support dose recommendation in specific population (e.g., patients with renal and hepatic impairment).
Mr. Tao Li, Senior Manager, Seattle Genetics, presented the third talk on “CMC Challenges and Opportunities in ADC Drug Development and Lifecycle Management”. Considering the uniqueness of the ADC drug’s molecular structure, the development of ADC products have been full of Chemistry, Manufacturing and Controls (CMC) challenges and uncertainties. From the Regulatory Affairs perspective, Mr. Li covered the brief history of the regulatory policy evolution from FDA and EMA about the ADC development. He further discussed details about the eCTD Module 3 structures; CMC challenges of mAb, drug-linker and conjugate drug substance (DS), as well as drug product (DP) in-use stability; and the commercial lifecycle management during the ADC clinical trials and its commercialization.
Dr. Pillow started with an overview of ADC chemistry including conjugation, linker and payloads. He discussed the challenge of heterogeneous conjugations and described Genentech's efforts to engineer Cys in the antibody so that drugs can be conjugated at a specific site, which results in homogeneous conjugates. Then Dr. Pillow mentioned the challenge of a narrow therapeutic index for cytotoxic ADCs and potential strategies to overcome this. Dr. Pillow showed an example of how to use an ADC to deliver a protein degrader into the target cells to improve the PK, efficacy and potentially safety of the small molecule. Dr. Pillow continued with another example of conjugating antibiotics to an antibody for anti-MRSA infection treatment.
Dr. Songqing Na, Research Fellow, Immunology Research, Eli Lilly, presented the last talk on “Antibody Drug Conjugate (ADC) Beyond Oncotherapies”. Dr. Na indicated that ADC could be used to deliver other types of payload for diseases beyond oncology, such as inflammation. His first example was about ADC with LXR agonist as payload that specifically targeted monocytes but not liver hepatocyte cells and showed improved anti-inflammation properties without affecting hepatocyte function. Dr. Na continued with another example, using glucocorticoid as payload to attach different antibodies for delivering different targeting cell types. From cytokine expression and GR gene regulation, Ab conjugated glucocorticoid showed potent anti-inflammation in targeted cells. In comparison to glucocorticoid treatment, the ADC greatly reduced the systemic effects of the small molecule drug. Finally, anti-TNF-Glucocorticoid conjugate showed superior efficacy in human RA patients compared with anti-TNF alone in a recent clinical trial.
We would like to thank all five speakers for their time and efforts for this webinar. We also acknowledge Ken Zhang, Xiang Yi, Sihong Zhou, Chenchao Gao, Jenen Tan, Yan Wang, Hesong Han, Guanghui Han, Carrie Wang, Yang Tian for organizing the event. To learn more about CABS, please visit our website www.cabsweb.orgOnline Zoom meeting
With the accelerated approval to Trodelvy in 2020, 9 antibody-drug conjugates (ADCs) now on the market have truly proven their potential. The Chinese American Biopharmaceutical Society (CABS) will host an ADC seminar on Aug 29, 2020 at 1:25-4:30pm. Brought together ADC experts from Seattle Genetics, Sutro Biopharma, Genentech, and Eli Lilly, the seminar will delve into the pioneering development of ADC from early discovery through to manufacturing CMC and clinical study. Join us to get exposed to ground-breaking ADC science and case studies and to network with ADC industrial professionals.
Date: August 29th 2020, Saturday, 1:25-4:30 PM PST
Registration not required. Please click the link below to join the webinar:
Agenda:
1:25 to 1:30 PM Welcome Remarks from CABS. Yang Tian, PhD, President, CABS.
1:30 to 2:15 PM Antibody Drug Conjugates - Using a Novel Technology to Produce First in Class Immune Modulating Cancer Drugs. Kristin Bedard, PhD, VP of Immuno-Biology, Sutro Biopharma, South San Francisco.
2:15 to 3:00 PM Clinical Pharmacology Strategies in Supporting Drug Development and Approval of Antibody Drug Conjugates. Chunze Li, PhD, Director/Principal Scientist/Group Leader, Oncology Clinical Pharmacology, Genentech, South San Francisco.
3:00 to 3:45 PM CMC Challenges and Opportunities in ADC Drug Development and Lifecycle Management. Tao Li, Senior Manager, Regulatory Affairs CMC, Seattle Genetics, Seattle.
3:45 to 4:30 PM Expanding the Application of Antibody Drug Conjugates through Chemistry. Thomas Pillow, PhD, Senior Scientist, Discovery Chemistry Department, Genentech, South San Francisco.
4:30 to 5:15 PM Antibody Drug Conjugate (ADC) Beyond Oncotherapies. Songqing Na, PhD, Research Fellow, Immunology Research, Lilly Biotechnology Center, Eli Lilly and Company, San Diego.
Speakers Background:

Dr. Kristin Bedard is the Vice President of Immuno-biology at Sutro Biopharma where her team is responsible for the preclinical development of immuno-oncology assets. She has over twenty years of experience in innate immunity, cancer research and drug development and was most recently the scientific co-founder and Chief Scientific Officer of Immuno-oncology at Kineta, Inc. where she led early discovery and development programs in infectious disease and oncology. Her team progressed the antiviral candidate, LHF-535, from discovery into clinical development for the treatment of Arenavirus infection. Additionally, she developed a proprietary innate immune screening platform and identified novel small molecule RIG-I agonists for the treatment of solid tumors which were licensed to Pfizer in 2017. Her work in innate immunity has been well published and she is the co-inventor on more than ten patents for novel immuno-oncology compounds and methods of identification. Prior to Kineta, Dr. Bedard was a senior fellow at the Fred Hutchinson Cancer Research Center where her research focused on viral induced skin and cervical cancers. She completed her PhD in molecular virology at the University of California, Irvine. Dr. Bedard volunteers her time as a board member for the Healthcare Businesswomen’s Association where she received the Everest Award in 2019 for her dedication and leadership. She also serves as chairwoman and scientific reviewer for the National Institutes of Health small business grant division.

Dr. Chunze Li is currently a Director/Principal Scientist/Group Leader in Oncology Clinical Pharmacology at Genentech. She oversees strategic clinical pharmacology support for diverse oncology molecules, including antibody-drug conjugates, Immune-cell bispecifics, cell therapy, hematological drugs, HER2 targeted therapies, and SC development for monoclonal antibodies. She is passionate about strategic implementation of quantitative clinical pharmacology to optimize the oncology drug development. Prior to Genentech, Dr. Li worked as Associate Director at Pfizer and as Research Fellow at Merck to provide Clin Pharm and DMPK support in drug discovery and development of small molecules, therapeutic proteins and monoclonal antibodies across multiple diseases. Dr. Li obtained her PhD degree in Pharmaceutical Chemistry at UCSF with Dr. Leslie Z. Benet. She has published over 50 peer-reviewed papers and book chapters in the field of clinical pharmacology, drug metabolism and pharmacokinetics.

Tao Li has more than 19 years of pharmaceutical industry experience in Regulatory Affairs CMC, drug discovery and development. Prior to Seattle Genetics, Tao worked at Abbott/AbbVie as a senior scientist focused on discovery and developing clinical candidates including new ADC payloads; most recently in RA CMC, with primary responsibility for post-approval lifecycle management of multiple marketed products including small molecule tablets, parenteral, and biologics. During his 1.5 years with Seattle Genetics, he played a key role in the Module 3 preparation, submissions and approvals for Tukysa™ NDA/MAAs. He is also responsible for multiple early stage programs as the RA CMC leader and has advanced three new biologics and antibody-drug conjugate (ADC) INDs/IMPDs into clinical trials. Tao holds a BSc in Chemistry and a MSc in Medicinal Chemistry from Shandong University. In addition, he has a graduate certificate of Regulatory & Quality Compliance from Purdue University.

Dr. Thomas Pillow is a Senior Scientist of the Discovery Chemistry Department at Genentech. He received his PhD from Stanford University in organic chemistry under the direction of Professor Paul Wender. Upon graduation he moved to Genentech in 2009 where he is currently a senior scientist in the discovery chemistry department leading Genentech’s effort on antibody-drug conjugates.

Songqing Na, PhD, is Research Fellow of the Immunology Research, Lilly Biotechnology Center, Eli Lilly and Co. in San Diego. Dr. Na received his PhD from Brandeis University in Boston. He was postdoctoral fellow at Harvard Medical School and then Pfizer/Yale Medical School. He joined Lilly research laboratory in Indianapolis in 1997, where he has been in research and drug discovery of Biotechnology, Inflammation, and Oncology. For the last 8 years, he has been working in immunology research and discovery in Lilly’s Biotechnology Center in San Diego. Dr. Na has published 37 peer-reviewed articles and two book chapters. Currently, Dr. Na is leading a Ph2a clinical trial team for a clinical asset.
Online Webinar through Zoom Conference
ChinaBio® Partnering Forum 2020
Delivered Digitally
August 25-27, 2020
CABS members enjoy 20% discount using VIP code ‘CABS20’
ChinaBio® Partnering Forum has grown to be the premier life science partnering conference in China since 2009. This year is our 12th anniversary, and for the first time will be delivered digitally! The conference will take place on August 25-27, using the powerful online platform provided by EBD Group, the largest provider of life science partnering events globally. Last year, there were over 1400 attendees from 25 countries who had over 3,200 1-1 meetings and saw nearly 80 company presentations during the 2-day conference. This year, going virtual will enable participants to hold meetings 24/7 using EBD Group's partneringONE® system, and to see the company presentations, keynote panels and workshops throughout the event. Join us this year for what will prove to be a dynamic and exciting event that will enable you to meet with colleagues from around the world, and to hear from executives, investors and industry experts from leading Chinese and global companies, as well as innovative startups.

Delivered Digitally
On August 22, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled “Contact Tracing and Control of COVID-19 Community Spread”. This webinar is co-organized with Chinese American Semiconductor Professional Association (CASPA) and Gracious Life Foundation (GLF). The webinar attracted almost 150 attendees from around the world. A replay of the webinar can be accessed HERE.
Dean Michael Lu, from UC Berkeley School of Public Health, started with a keynote speech on “Innovating Solutions to Covid-19”. Dr. Lu started with an overview of reentry testing such as saliva testing, pooled testing, and CRISPR-based point-of-care mobile testing, as well as outbreak surveillance technologies such as adaptive surveillance using sewage, water and air sample testing. Then Dr. Lu discussed infection control strategies, such as facility modifications, behavior modification and digital contact tracing. Dr. Lu also discussed instructional redesign strategies to prepare for remote/hybrid classroom learning. In the end, Dr.Li discussed ways to prevent future pandemics, such as preventing zoonotic transmission, global early warning system and AI-powered drug discovery. “The real solution will come by transdisciplinary, transinstitutional, and transnational collaborations”, Dr. Lu concluded his talk.
Dr. James Mu, from Providence Regional Medical Center, Seattle, Washington, presented “COVID-19 contact tracing, therapeutics and vaccine development”. Dr. Mu explained the power of genomic (phylogenetic) tracing in addition to the traditional contact tracing. He also reviewed the literature on chloroquine, remdesivir and dexamethasone for COVID-19 treatment. Dr. Mu then discussed established (inactivated virus and recombinant vaccines) vs. new nucleic acid vaccine development strategies and the respective human clinical trial data. COVID-19 spike protein is the common target antigen used by most COVID-19 vaccines in development, but Dr. Mu pointed out that purified spike protein and live virus behave differently, making vaccine development more challenging. Dr. Mu is, however, cautiously optimistic in a successful vaccine for COVID-19.

Professor Zuo-Feng Zhang, from UCLA Fielding School of Public Health, presented “Epidemiology and Control of COVID-19 Pandemics: Current Status & Future Trends”. Dr. Zhang started with an overview of COVID-19 epidemiology statistics around the world, such as US, Brazil, and India. In the US, we started to see the new reported cases going down in last 2-3 weeks. “This is a good sign”, Dr.Zhang said, “it is also encouraging to see it now takes longer to accumulate 1 million new cases than before, this is indicating the spread is slowing down”. Dr. Zhang also demonstrated that mortality rate is much higher in non-Asian countries than in Asian countries, possibly due to the use of face masks, BCG vaccination, fewer co-morbidities, sufficient testing or over/under reporting deaths.

Our last speaker, Professor Po-Shen Loh, from Carnegie Melon University, presented “An alternative paradigm for app-based contact tracing which incentivises adoption”. “COVID-19 is a network theory”, Dr. Loh said, "for example, the R0 number in COVID-19 is related to measures of connectivity in network structure". Then Dr. Loh demonstrated how a mobile App “NOVID”, which he helped designed, could be used to encourage social distancing especially in college. NOVID can show how the spread of COVID-19 relates to you, not in terms of physical distance but in terms of degree of connection. “This is more effective than a case heatmap by zip code”, Dr. Loh said, “it can really tell you whether the disease is approaching you or not, then you can modulate your behavior”.
Finally, Dr. Michael Prelip, Professor and Chair, Department of Community Health Sciences, UCLA, moderated a panel discussion among the four speakers and discussed important questions related to disparity in healthcare access and strategies to encourage behavioral change and vaccination.
We would like to thank all five speakers for their time and contribution. We also want to thank Jenen Tan, Hesong Han, Guanghui Han, Carrie Wang, Weixing Chen, Song Xue for organizing the logistics of the event. To learn more about CABS’ effort in the COVID-19 response, please visit our website www.cabsweb.org.
https://zoom.us/rec/share/w_1FBKrJzWBOcJXg6GOceaJ7R4rOaaa8hCYd_fsEnhsT97NQP0pjJyEBpZWyQh0u
CABS-GLF-CASPA Webinar
Contact Tracing and Control of COVID-19 Community Spread
The COVID‑19 pandemic, is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2). As of August 10th, 2020, approximately 20.1 million confirmed COVID-19 cases, and more than seven hundred thousand deaths had been attributed to COVID-19 worldwide.
The COVID-19 pandemic has caused global social and economic disruption, including the largest global recession since the Great Depression. As the world reopens, we continue to face challenges in controlling the community spread of coronavirus. What are the current status and further trends? Comparison of the COVID-19 situation in Massachusetts (East Coast) and California (West Coast)? How do some heavily-populated countries like India and Brazil fight the disease? How to control coronavirus community spread? How should we do contact tracing? What’s the current progress in medical therapeutics and vaccine development? What hi-tech innovative solution can help to fight this war?
Please join us in the upcoming webinar “Contact Tracing and Control of COVID-19 Community Spread” on Saturday, August 22nd, 2020, 2:00 – 4:30 PM PST. CABS has partnered with Gracious Life Foundation (GLF), Chinese American Semiconductor Professional Association (CASPA), to organize this exciting event. We have invited a panel of distinguished speakers from public health, clinical medicine, and big data analytics.
Agenda (August 22, 2020, Saturday; Agenda is based on US Pacific Time)
2:00 pm - 2:10 pm Introduction:
Introduction to CABS, Yang Tian, President of CABS
Introduction to CASPA, Song Xue, President of CASPA
Introduction to GLF, Weixing Chen, President of Gracious Life Foundation
2:10 pm - 2:40 pm Innovating Solutions to Covid-19, Michael C. Lu, MD, MS, University of California, Berkeley
2:40 pm - 3:10 pm Progress in COVID-19 Therapeutic/Vaccine Research , James Mu, MD, PERMC and WWMG, Seattle, WA
3:10 pm - 3:40 pm Epidemiology and Control of COVID-19 Pandemics: Current Status & Future Trends, Zuo-Feng Zhang, MD, PhD, University of California, Los Angeles
3:40 pm - 4:05 pm An alternative paradigm for app-based contact tracing which incentivises adoption, Po-shen Loh, PhD, Professor, Carnegie Melon University
4:05 pm - 4:30 pm Panel Discussion moderated by Michael Prelip, PhD, Professor and Chair, Department of Community Health Sciences, University of California, Los Angeles
Due to large number of registrants, please join the webinar 5 minutes before the starting time (2 pm US Pacific Time). Please email for any question: info@cabsweb.org
Speakers
Michael C. Lu, MD, MS, MPH.
Dean, School of Public Health, University of California, Berkeley
Dr. Michael C. Lu is formerly a bureau director for maternal and child health in the Obama Administration and also professor of ob-gyn and public health at UCLA. He is the current Dean of the UC Berkeley School of Public Health, has a deep-rooted passion for health equity and social justice. He has dedicated his research to the development, testing and translation of a new theory on the origins of maternal and child health disparities.
.James Mu, MD.
PERMC and WWMG, Seattle, WA
Dr. James Mu received his undergraduate medical education and his first medical residency and GI fellowship in Chengdu, China then received clinical residency and GI fellowship training at Washington University in St. Louis. He also completed research GI fellowship under Drs. Isenberg at UCSD and Alpers at Washington University. As a postdoctoral research fellow, he received " individual National Research Service Award (NRSA)" from NIH and "Young Investigator's Award" from Federation of American Societies for Experimental Biology (FASEB). In 2000 he joined Western Washington Medical Group and Providence Regional Medical Center in Everett. He has been rated as a Top Doctor several times since 2009 by Seattle Metropolitan magazine and by Seattle Magazine.
Zuo-Feng Zhang, MD, PhD.
Distinguished Professor of Epidemiology Associate Dean for Research Fielding School of Public Health,University of California, Los Angeles
Dr. Zuo-Feng Zhang is a Distinguished Professor of Epidemiology and Associate Dean for Research of the UCLA Fielding School of Public Health. He is also Co-Director of the UCLA Alper Center for Environment Genomics, and Director of Cancer Molecular Epidemiology Training Program. Dr. Zhang had been a Fellow of the American College of Epidemiology (ACE). He served a member of the Board of Directors of the American College of Epidemiology and a World Health Organization (WHO) Consultant for National Non-communicable Disease Prevention and Controls in China.
Po-shen Loh, PhD.
Professor, Department of Mathematics, Carnegie Melon University
Professor Po-shen Loh is a social entrepreneur, working across the spectrum of mathematics: from education to pure and applied research. He is a math professor at Carnegie Mellon University, and the founder of the free personalized learning platform expii.com, a social enterprise supported by his own Daily Challenge series of online middle school math courses that reinvent middle school math for students seeking more than the standard USA curriculum. He is also the national coach of the USA International Mathematical Olympiad team which ranked #1 in the world 4 times in the last 5 years. During the COVID-19 outbreak, he turned his mathematical attention to creating NOVID, the world's first (and still only) COVID-19 app demonstrably capable of measuring distance with the requisite accuracy for contact tracing.He has been recognized by various awards, from an International Math Olympiad silver medal to the USA Presidential Early Career Award for Scientists and Engineers.
Michael Prelip, DPA.
Professor and Chair, Department of Community Health Sciences, University of California, Los Angeles
Dr. Mike Prelip is currently a professor and chair of the Department of Community Health Sciences (CHS) at the University of California Los Angeles (UCLA) Fielding School of Public Health. He was also the School’s first Associate Dean of Practice and for many years the director of the CHS MPH for Health Professionals Program. He is currently involved in a number of translational research projects, which are all community based and public health focused, using a community engagement model. Dr. Prelip conducts his work in both domestic and international settings. He has received numerous prestigious National Institute of Health awards to support his research, including multiple awards from the National Institute of Child Health and Human Development, the National Heart Lung Blood Institute, the National Institute of Nursing Research and the Fogarty Center. He also directs a number of NIH and CDC funded training programs focusing on health disparities. Recently he was awarded a $2.7 million grant from the CDC to establish FSPH’s Undergraduate Public Health Scholars Program. In response to the COVID-19 pandemic, Dr. Prelip is co-leading UCLA’s partnership with UC San Francisco to train over 10,000 COVID-19 contact tracers and case investigators for the California Department of Public Health being deployed throughout California. He is also working with the Andean Parliament (consortium of Bolivia, Peru, Colombia, Ecuador, and Chile) to create similar trainings for these South American countries.
Online Webinar through Zoom Conference
Free Online Symposium: Diagnostic Tools and Therapeutics for COVID-19: An Overview and Forward Analysis
Date/Time: Aug 18, 2020; 9:00 - 11:00
Venue: Online via Zoom by PBSS-SF Bay
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Delivered Digitally
Q Bay Global Pitch Day
Date & Time:
6:00 pm-8:30 pm, Wednesday, July 29 (PDT, US)
Topic: Medical device
Venue: Q Bay Training Center via Zoom, presented to the US, China, and Japan.
Attendees: 8-10 startups based in Silicon Valley or Boston, 20+ investors from Q Bay global network.
Agenda:
6:00 pm-6:05 pm, Opening remark.
6:05 pm-8:05 pm, Pitch and Q & A from 8-10 Startups.
Each startup has 10 minutes to pitch their idea through video and we will leave 5 minutes after the pitch for our investors to ask questions and make interaction.
8:05 pm-8:30 pm, Open Q & A session from all investors/partners/attendees.
We welcome startups who have a clear business plan and an international market demand to join us on this virtual event.
You will get
⁃ 15 minutes to present your concepts to our online audience of investors and industrial partners.
⁃ Free workspace and VIP membership at Q Bay Center for 3 months.
⁃ Access to capital, mentorship and consulting in your market.
⁃ Access to industry training events.
Startup registration: https://forms.gle/PWuYdW3mbC1EHSQL7
About Q Bay Center
Q Bay Center, founded by Hangzhou Overseas Center Inc., is a Silicon-Valley-based platform to connect and support innovation and entrepreneurship. With a tripartite mission of “Platform + Investment + Services”, Q Bay Center is designed as an innovative business model which combines real property, business exhibition, financial activity, technology cooperation, business services and startups incubation. By leveraging the synergy of technology, finance, industrial ecology and culture, the center is committed to enhancing connection and empowering development.
Delivered Digitally
Topic
Future of public health & role of global partnership (by US Heartland China Association)
Description
Even before the current pandemic, the Heartland region faced significant public health challenges, such as the opioid epidemic. CoVID-19 further exposed weaknesses in our public health system and accelerated the adoption of new ways to provide care.
In this event, we will hear public health experts, industry leaders and practitioners share their thoughts on the future of public health and how global collaboration can shape an outcome beneficial to us all.
Time: Jul 28, 2020 09:00 AM in Eastern Time (US and Canada)
Registration link:
https://us02web.zoom.us/webinar/register/6715945622803/WN_woeMStyvRviG8R7PZNnX1g
Delivered Digitally (Zoom)

CABS Webinar
Science and Technology Caught Between the United States and China
US Pacific Time: June 27, 2020, 4:00pm-5:30pm,
China Beijing Time: June 28, 2020, 7:00am-8:30am

Zoom Link https://zoom.com.cn/j/96237222412
Or join by phone:
Dial(for higher quality, dial a number based on your current location):
US: +1 669 900 9128 or +1 253 215 8782 or +1 346 248 7799 or +1 646 558 8656 or +1 301 715 8592 or +1 312 626 6799
Webinar ID: 962 3722 2412

This webinar is organized by CABS and co-organized by BayHelix. It will feature:
•New Era of U.S.-China Relations and Challenges for Chinese-American STEM Professionals
•Economic Espionage and NationalSecurity
•Against Racial Profiling:
- Dr Wen Ho Lee and his successful civil lawsuit against the US government
- Rodney King incident and Christopher Commission
•Navigating Career Opportunities and Avoiding Pitfalls in the 21st Century of U.S.-China Relations

Free Registration by clicking here or "Register" button at bottom
Or
Free Registration at Eventbrite, click here to register.
Zoom access link will be sent to your registration email.

Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
4:00pm - 4:05pm Opening Remarks
4:05pm - 5:10pm Science and Technology Caught Between the United States and China, Brian A. Sun, Jones Day.
5:10pm - 5:30pm Q&A

Speaker

Brian A. Sun
Brian Sun is former president of the National Asian Pacific American Bar Association (NAPABA) and Of Counsel of law firm Jones Day in Los Angeles office. In 2013 he was honored by NAPABA with its Trailblazer Award and by the Southern California Chinese Lawyers Association with its Lifetime Achievement Award. He also served as a deputy general counsel to the Christopher Commission, which recommended sweeping reforms of the Los Angeles Police Department in the wake of the Rodney King incident. He represented Dr Wen Ho Lee in his successful civil lawsuit against the US government.
Brian Sun has earned a national reputation as a distinguished trial lawyer in complex business litigation and white collar criminal defense. He is a former federal prosecutor, a Fellow of the American College of Trial Lawyers, and has been named by Lawdragon Magazine as one of America's 500 leading lawyers. Brian is recognized as a Band 1 lawyer by Chambers and listed among the most highly regarded lawyers in the U.S. by Who's Who Legal in the area of business crime defense.
Recommend Using PC/Mac to Register. If CANNOT register using an iPhone, click here.
You can also register free at Eventbrite, click here to register.
Zoom Conference
On June 27, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled “Science and Technology Caught Between the United States and China”. Brian Sun from law firm Jones Day presented the talk. This webinar attracted more than 500 attendees from around the world
Reply of of the webinar can be accessed here

Dr. Huijun Zhou, Chair of CABS Entrepreneurship Club, started the webinar with an introduction of Brian Sun.
Brian Sun is former president of the National Asian Pacific American Bar Association (NAPABA) and Of Counsel of law firm Jones Day in Los Angeles office. In 2013 he was honored by NAPABA with its Trailblazer Award and by the Southern California Chinese Lawyers Association with its Lifetime Achievement Award. He also served as a deputy general counsel to the Christopher Commission, which recommended sweeping reforms of the Los Angeles Police Department in the wake of the Rodney King incident. He represented Dr Wen Ho Lee in his successful civil lawsuit against the US government.
Brian Sun has earned a national reputation as a distinguished trial lawyer in complex business litigation and white collar criminal defense. He is a former federal prosecutor, a Fellow of the American College of Trial Lawyers, and has been named by Lawdragon Magazine as one of America's 500 leading lawyers. Brian is recognized as a Band 1 lawyer by Chambers and listed among the most highly regarded lawyers in the U.S. by Who's Who Legal in the area of business crime defense.
In the presentation, Brian started with a historical review on the bias on Chinese-American and current new era of U.S.-China Relations and Challenges for Chinese-American STEM Professionals. Then Brian talked about three racial profiling cases, and how those cases promoted Chinese-Americans to fight for their rights since 1980s. Brian followed his talk with a few recent cases against Chinese-American STEM professionals working at US academic institutes and private companies. In the end Brian gave some practical advices on how to navigate career opportunities and avoiding pitfalls in the 21st Century of U.S.-China relations, including U.S. criminal statues and defenses, and best practices for companies and institutions. “All Chinese-Americans need to get organized, get their acts together, work together and speaker up, to protect our future generation, to improve ourselves, and to improve our country”, Brian concluded this talk.
After the presentation, Brian answered questions from audience.
We would like to thank Brian Sun for his time and efforts for this webinar. We also want to thank Tony Chen and BayHelix for co-organizing this webinar; thanks to Huijun Zhou, Yang Tian, Hesong Han, Jenen Tan, Guanghui Han, and Carrie Wang, for organizing the logistics of the event. To learn more about CABS’ events and its effort in the COVID-19 response, please visit our website www.cabsweb.org. A replay of the webinar recording will be available on this site.
Online
Bio S&T is co-founded by CCCMHPIE, UBM Sinoexpo and Bosnan Consulting, and has deep collaboration with global biotech professional organizations, biopharmaceutical industry and seasoned financial investors interested in biotechnology. It serves as a new platform for the introduction & exchanges of new development in biopharmaceutical science and technologies.
Link: www.bio-St.com
Shanghai New Int'l Expo Center

Challenges After Re-opens: Will Massive Testing Be Our Safeguard?
US Pacific Time: Jun 20th, 2020, 2:00pm-4:30pm
Click HERE to see the webinar recording
On June 20, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted the webinar “challenges after re-opens: will massive testing be our safeguard? ”. The event was co-organized by the Gracious Life Foundation (GLF). Six invited panelists include industry leaders from Bio-techne, BGI Group, Twist Bioscience, as well as academic researchers from University of Texas, Carnegie Mellon University, and Johns Hopkins University. Presentations and panel discussions focused on existing and innovative tests, including high throughput PCR testing, antibody testing and point-of-care testing capabilities, and their potentials on achieving widespread availability of rapid, efficient COVID-19 diagnostics. The webinar attracted more than 130 life sciences professionals to attend.

Quan-Zhen Li, MD, Ph.D.
Associate professor, University of Texas
Dr. Li outlined the current state of molecular and immunological testing for SARS-CoV-2, including nucleic acid, antigen, and antibody testing methods. He also described the possibility of using protein arrays as a multiplex platform for testing numerous antigens in parallel with high sensitivity.
Emily Leproust, Ph.D.
CEO and co-founder, Twist Biosciences
Dr. Leproust gave an overview of products from Twist Biosciences that are dedicated to the testing and diagnosis of SARS-CoV-2. These included synthetic viral RNA controls for validation of diagnostic tests, a SARS-CoV2 research panel for NGS target enrichment to track mutations in the CoV2 genome, and an immunological assay for S1 and ACE2 proteins using antibodies discovered in-house.
Brendan Yee
Director of Immunoassays, Bio-Techne
Mr. Yee described the SARS-CoV-2 serology test that Bio-Techne will be manufacturing through their partnership with Mount Sinai Hospital and Kantaro. The test, a two-phase ELISA assay to assess presence of two proteins unique to SARS-CoV2, was developed by the Icahn School of Medicine and has been performed on over 40,000 SARS-CoV2 patients at Mount Sinai. He also mentioned Bio-Techne’s ELLA Cytokine Storm Panel, an automated ELISA system that will measure cytokine levels in patients to determine when their disease has reached a critical point.
Liben Chen, Ph.D.
Associate Research Scientist, Johns Hopkins University
Dr. Chen detailed the magneto fluidics based PROMPT system, designed to address many of the problems present in SARS-CoV-2 testing today. PROMPT would utilize automated qPCR on microfluidics cartridges, providing diagnosis results in just 15-20 minutes while maintaining a very low cost. It is currently pending EUA approval, and will hopefully deploy to various departments in the Johns Hopkins medical system this summer.
Yongwei Zhang, Ph.D.
VP & CEO of Americas, BGI Group
Dr. Zhang discussed BGI’s various solutions and efforts for SARS-CoV2 testing. BGI’s first diagnostic RT-PCR kit was created in Wuhan just 1-2 weeks after the initial outbreak, and was one of the first such tests ever developed. Their other products include the MGISP-869, an automated system using magnetic beads to extract DNA from patient samples.
Laurence Ales, Ph.D.
Associate Professor of Economics, Carnegie Mellon University
Dr. Ales spoke on the global economic repercussions of the COVID-19 pandemic, especially in regards to its impact on consumer behavior. Due to the colossal effect of COVID-19 on the lives of individuals worldwide, pandemic fears will likely play a large role in the economies of countries such as the United States for the foreseeable future. Dr. Ales is currently leading a large scale study to elucidate these effects and determine a viable road to economic recovery.
We sincerely thank all the speakers, GLF co-organizer (Weixin Chen), CABS volunteers, Zoom and the Beijing WellBeing Foundation for their time and support.



Speakers' Bio

Quan-Zhen Li, MD, PhD
Associate professor of Department of Immunology and Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
Dr. Li obtained his Medical Doctor’s degree from the Third Military Medical University in 1984 and PhD degree from Sun Yet-Sun University in 1993. He had his post-doctoral training in Depart of Pathology, University of Florida (1999-2001) and Department of Immunology, University of Texas Southwestern Medical Center (UTSW) (2001-2004). Dr. Li joined the faculty of UTSW in 2004 and now is an Associate professor of Department of Immunology and Internal Medicine of UTSW. He served as the co-director of the Microarray and Genomics Core facility in UTSW since 2004. His main research focuses are the genetics and molecular mechanisms on autoimmunity and autoimmune diseases. He has published over 100 peer reviewed research articles and reviews.

Brendan Yee,
Director, Immunoassay, Bio-Techne
Experienced biotechnology leader with over 30 years of experience directing product development and commercialization efforts to support genomic, proteomic, and cellular research in the life science and clinical markets. Prior to directing Bio-Techne’s efforts for their various immunoassay platforms, Brendan directed the development and commercialization of several familiar research platforms at Affymetrix, Beckman Coulter, and Bio-Rad Laboratories Brendan will discuss the automated immunoassay platform offered by Bio-Techne that is actively supporting COVID-19 research for patients that are affected by Cytokine Storm.
 Emily Leproust, Ph.D.
Emily Leproust, Ph.D.
CEO and co-founder, Twist Bioscience
As an early pioneer in the high-throughput synthesis and sequencing of DNA, Dr. Leproust is disrupting the process of gene synthesis to enable the exponential growth of synthetic biology applications in multiple fields including medicine, DNA data storage, agricultural biology, and industrial chemicals. In 2015, she was named one of Foreign Policy’s 100 Leading Global Thinkers for fast-tracking the building blocks of life, and Fast Company named her one of the most creative people in business for synthesizing DNA faster than ever. Prior to Twist Bioscience, she held escalating positions at Agilent Technologies where she architected the successful SureSelect product line that lowered the cost of sequencing and elucidated mechanisms responsible for dozens of Mendelian diseases. She also developed the Oligo Library Synthesis technology, where she initiated and led product and business development activities for the team. Dr. Leproust designed and developed multiple commercial synthesis platforms to streamline microarray manufacturing and fabrication. Prior to Agilent, she worked with Dr. X. Gao at the University of Houston developing DNA and RNA parallel synthesis processes on solid support, a project developed commercially by Xeotron Corporation. Dr. Leproust has published over 30 peer-reviewed papers—many on applications of synthetic DNA, and is the author of numerous patents. She earned her Ph.D. in organic chemistry from the University of Houston and her M.Sc. in industrial chemistry from the Lyon School of Industrial Chemistry in France.
Yongwei Zhang, Ph.D.
BGI Group VP & CEO of Americas
Yongwei serves as Group VP of BGI-Shenzhen (华大集团副总裁), one of world’s largest genomics service companies. He is also CEO of BGI Americas Region and leads BGI’s COVID-19 related businesses in Americas. Dr. Zhang has over 20 years of experience in optics-based instruments and systems. Prior to BGI, he worked in various high tech startups in Bay Area including one he co-founded. Dr. Zhang received his B. Eng in Precision Instruments and B.Sc. in Applied Mathematics from Tsinghua University in Beijing, M.S. in Computer Sciences, M.S. and Ph.D. in Mechanical Engineering from The Johns Hopkins University in Baltimore, MD.

Liben Chen, Ph.D.
Associate Research Scientist at Johns Hopkins University
Dr. Liben Chen is an Associate Research Scientist at Johns Hopkins University, Baltimore, MD. Dr. Chen received his Doctor of Veterinary Medicine degree from Huazhong Agricultural University in 2005 and obtained his Ph.D. degree from China Agricultural University in 2010. Prior to Johns Hopkins University, he worked at the Beijing Center for Animal Disease Control and Prevention, where his primary work was to develop rapid diagnostic tools for animal infectious diseases. In 2015, Dr. Chen joined the research faculty of Johns Hopkins University Whiting School of Engineering, where his research focuses on developing technology platforms for fast diagnosis of bacterial and viral infections.
 Laurence Ales, Ph.D.
Laurence Ales, Ph.D.
Associate Professor of Economics, Tepper School of Business, Carnegie Mellon University
Dr. Laurence Ales is an Associate Professor of Economics at Carnegie Mellon University’s Tepper School of Business. His research focuses on the study of inequality and the design of tax policy. Recent work has analyzed how to tax the labor income of highly paid CEOs and how the income tax schedule should change over time due to polarizing technical change. Current projects study the impact that future disruptive technologies will have on labor markets and determine the best response of policy makers in the form of tax subsidies and migration assistance. Ales joined Carnegie Mellon University in 2008. Before joining Carnegie Mellon, Ales completed his PhD in economics at the University of Minnesota and attended the University of Rome, Tor Vergata, where he received his bachelor’s degree in Physics.


Zoom Conference
Executives Roundtable: Career Advancement & Leadership
US Pacific Time: Jun 14th, 2020, 5:00pm-6:30pm
Register on Eventbrite (managed by IntelliPro)
to receive Zoom link


The webinar held by Intellipro Group and CABS (Chinese American Bio/Pharmaceutical Society) on June 14, 2020 invited four distinguished guest speakers including Dr. David Gravett , VP of strategy at Poly-Med Inc., Dr. Jen Majeti, head of global collaborations and general manager (China) at Erasca Inc., Dr. Selena Yuan, director of global talent management at Gilead sciences Inc. and Dr Zhenhai Shen, CEO of NewBay pharma Inc.
They shared views about major areas of career transitions from R&D to business operations, the impact of COVID-19 on business operations and management, as well as the differences between working in big corporations and small companies or sartups. The webinar was moderated by Ross, the strategic partnership leader of IntelliPro Group and was processed in the form of Q&A.
The Transition from Realm of Scientific Research to the Real Business World
Dr. Zhenhai Shen: Shifting from academia to the real business settings could be difficult. You need to adapt yourself to a completely new environment and explore from scratch. However, if you’ve already worked in a company, let’s say for example the RD department, it will be easier for the move because you have the opportunities to learn through cross-function rotations and communication with your co-workers.
The biggest challenge is to change the way you work- from a hard-skill oriented and self-presenting perspective to soft skill acquiring like negotiating skills and network building.
The Impact of COVID-19 on Business Operations and Management
Dr. Selena Yuan: As the overall level of education keeps increasing, leadership capability becomes a key point for you to signal yourself in the labor market. Besides some soft skills mentioned by Zhenhai, emotional intelligence is what management can benefit from and should constantly improve that it’s the motivation behind behaviors that could influence the whole team.
Dr. Jen Majeti: Communication, presentation and establishment of relationships are critical skills for people, especially those who are at senior levels.
Criteria for Evaluating Setups
Dr. Jen Majeti: For leadership-ability to raise fund, ability to recruit talent, and ability to execute. The level of the match with the market.
Small Setups vs. Big Corporations-Which to Choose?
Dr. David Gravett: Pros of small companies- good platform for teamwork, facilitating multitasking skills and the gaining of a big picture of the company.
Pros of big companies- training for professionals in specific areas and a lot of resources and opportunities for self-development.
Dr. Selena Yuan: Be aware of your expectation in certain phases of your career and then make the right choice. Small companies may be risker to some extent, but at the same time it can serve as a good preparation and practice before you step into a large corporation.
Dr. Zhenhai Shen: Systemic training provided by big companies is an advantage.
Conflict Resolution
Dr. Zhenhai Shen: Truly listen to other people’s views and don’t prejudge.Don’t focus on disagreement, focus on concerns.
Dr. Jen Majeti: Listening clarifying and negotiation skills are important.React proactively,define the conflict, analyze the conflict of its root cause, and discuss your solution with your manager to get support.
Dr. David Gravett: Communicate early and communicate a lot, boil down to facts, and be objective, document the communication and clarify often.
Dr. Selena Yuan: Choose the right time and manner to deliver your messages.
To sum up, we are living in challenging times and challenges always bring opportunities. Companies that are willing to have the flexibilities to embrace changes and have global perspectives will gain competitive advantages over their competitors.




 Free registration at Eventbrite (managed by Intellipro) and is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.
Free registration at Eventbrite (managed by Intellipro) and is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.

About IntelliPro:
Founded in 2009, IntelliPro Group Inc. is one of the fastest-growing IT services and HR solutions companies serving all industries in the Americas & APAC. We provide a scalable continuum of services including IT talent solutions, workforce management strategies, and deliverable-based solutions designed to empower your business. Our client portfolio ranges from Fortune 500 companies and top-tier tech firms to innovative start-ups. By leveraging AI, big data, and network effects, IntelliPro Group Inc. has built its database for talent matchmaking.
About CABS:
The Chinese American Biopharmaceutical Society (CABS) is a non-profit organization for professionals in the biopharmaceutical industry. CABS is headquartered in San Francisco Bay Area, California. CABS is a highly influential association with more than 3000 members and subscribers in the life sciences industry. About 70% of our members have PhD degree relating to life sciences. A considerable proportion of the members are holding senior research and management positions in American and multi-national life sciences corporations. Many of our members are experts and leaders, innovative entrepreneurs, lawyers and venture capitalists or investors in the life sciences sector. CABS is the largest and most active Chinese biopharmaceutical association in Northern America.
Zoom Conference

Fireside Chat with Dr. John Wang
The Race for COVID-19 Vaccines
US Pacific Time: May 16, 2020, 4:00pm-5:00pm,
China Beijing Time: May 17, 2020, 7:00am-8:00am

•Latest updates on COVID-19 vaccine R&D
•Which vaccine technology likely to win the race?
•When will a COVID-19 vaccine be ready?
•What are the challenges in COVID-19 vaccine development
•Many more…

Join from a PC, Mac, iPad, iPhone or Android device:
Please click this URL to join. https://zoom.com.cn/j/96748683017
Or join by phone:
Dial(for higher quality, dial a number based on your current location):
US: +1 301 715 8592 or +1 312 626 6799 or +1 346 248 7799 or +1 646 558 8656 or +1 669 900 9128 or +1 253 215 8782
WebinaJoin from a PC, Mac, iPad, iPhone or Android device:
Please click this URL to join. https://zoom.com.cn/j/96748683017
Free Registration by clicking here or "Register" button at bottom
Or
Free Registration at Eventbrite, click here to register.
Zoom access link will be sent to your registration email.

Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
4:00pm - 4:05pm Introduction to CABS, Dr. Yang Tian, President of CABS
4:05pm - 5:00pm Fireside Chat with Dr. John Wang: The Race for COVID-19 Vaccine

Speaker

John (Jianxin) Wang, PhD
Dr. John Wang graduated from China Pharmaceutical University and received his PhD in Pharmacology from Mario Negri Institute of Pharmacological Research, Italy and postdoc training at University of Arizona at Tuson, US. In 1988, John joined the Department of Molecular Immunology of the Commonwealth Serum Laboratories (CSL), Australia and then its spin-off company "Mimotopes". John was transferred to the US company Chiron in 1995 and then Novartis in 2006, as associate director of Drug Discovery.
While in US, John joined the International Society for Biomolecular Screening (SBS) In 1996, and served as the Chair for both regional and International Annual Conferences, and later two terms of its Board member and President-elect before the society merged into the Society of Laboratory Automation and Screening (SLAS). In 1998, Dr. Wang also co-founded the Chinese-American BioPharmaceutical Society (CABS).
John returned to China and founded ImmuOn Therapeutics in 2012 to focus at research and development of therapeutic vaccines for chronic infection and cancer. In 2018 the cancer vaccine business was spun-off to become IOVaxis Therapeutics. The company currently has two cancer vaccine IND applications waiting for approval by NMPA.
Since 2014, John has been invited by the China Medical Economic News to be the columnist for the column "Immune, Gene and Health" . He has so far published over 200 articles.
Recommend Using PC/Mac to Register. If CANNOT register using an iPhone, click here.
You can also register free at Eventbrite, click here to register.
Special Thanks to Zoom Video Communications and Beijing Well-being Foundation for technical support.

Zoom Conference
On May 16, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled
“Fireside Chat with Dr. John Wang: The Race for COVID-19 Vaccines”.
By Dr. John Wang, CEO of ImmuOn Therapeutics
A replay of the webinar can be accessed here

The webinar attracted almost 300 attendees from around the world
(see attendees distribution below)
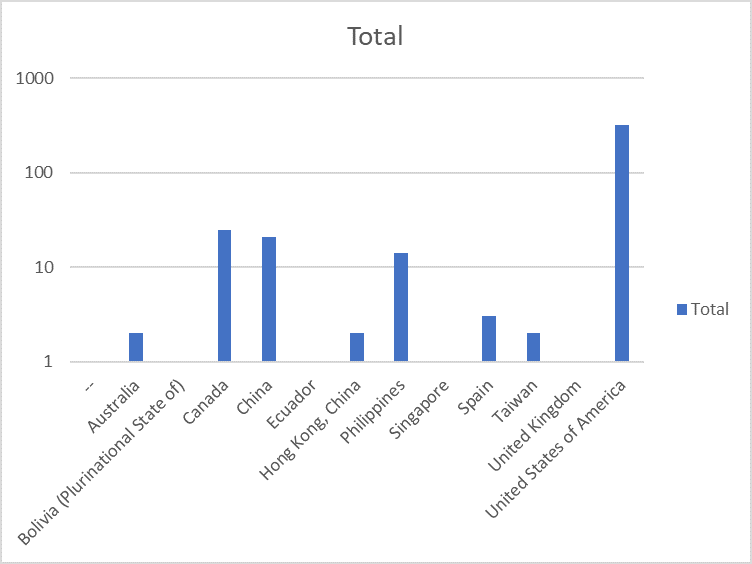
Dr. John Wang started his research career at the Department of Molecular Immunology of the Commonwealth Serum Laboratories (CSL), Australia and then its spin-off company "Mimotopes". He joined US company Chiron in 1995 and then Novartis in 2006, as Associate Director of Drug Discovery.
John returned to China and founded ImmuOn Therapeutics in 2012 to focus at research and development of therapeutic vaccines for chronic infection and cancer. In 2018 the cancer vaccine business was spun-off to become IOVaxis Therapeutics. The company currently has two cancer vaccine IND applications waiting for approval by NMPA. Since 2014, John has been invited by the China Medical Economic News to be the columnist for the column "Immune, Gene and Health". He has so far published over 200 articles.
While in US, John joined the International Society for Biomolecular Screening (SBS) In 1996, and served as the Chair for both regional and International Annual Conferences, and later two terms of its Board member and President-elect before the society merged into the Society of Laboratory Automation and Screening (SLAS). In 1998, Dr. Wang also co-founded the Chinese-American BioPharmaceutical Society (CABS).
Dr. Yang Tian, President of CABS, started the webinar with an introduction of CABS and its efforts for combating COVID-19, including a series of scientific webinars and a PPE donation campaign.
Dr. Wang then started his talk on why we need COVID-19 vaccines, followed by a review of five major vaccine technology platforms and current major players in the field. Then Dr. Wang discussed clinical trials of COVID-19 vaccine, specifically the phase 3 challenge trail. Further on, Dr. Wang talked about potential risk factors of developing a COVID-19 vaccine, including ADE. In the end, Dr. Wang discussed current status of COVID-19 diagnostics and epidemiology features. In the end, Dr. Wang expressed his optimism on developing an safe and effective COVID-19 vaccine based on current technology we have.
There were 3 poll surveys from the audience during the webinar.
The first survey question was “which vaccine technology will likely give the 1st COVID-19 vaccine approved?”
l 31% of attendees chose “inactivated or attenuated viruses”,
l 28% of attendees choose “mRNA vaccines”.
The 2nd survey question was “when will the 1st COVID-19 vaccine be likely approved?”.
l 45% of attendees chose by June 2021,
l 36% of attendees chose byDecember, 2020.
The 3rd survey question was “would you take the COVID-19 vaccine if it is approved and available?
l 65% of attendees chose “yes”
l 35% of attendees chose “No”.
We would like to thank Dr. John Wang for his time and efforts for this webinar. We also want to thank Jenen Tan, Hesong Han, Guanghui Han, Carrie Wang, for organizing the logistics of the event and thanks to Zoom and Beijing Well-being Foundation for providing technology support for this webinar.
To learn more about CABS’ effort in the COVID-19 response, please visit our website www.cabsweb.org.
A replay of the webinar recording will be available on this site.
Online

CABS Webinar
Stress Relief and Mental Health in COVID-19 Pandemic
US Pacific Time: May 9, 2020, 2:00pm-3:00pm,
China Beijing Time: May 10, 2020, 5:00am-6:00am

•Healthy Relationship with Spouse, Children and Parents
•Coping with Anxiety
•Work from Home with Kids
•Prepare for Reopening

Please click this URL to join. https://zoom.com.cn/j/94446901740

Free Registration by clicking here or "Register" button at bottom
Or
Free Registration at Eventbrite, click here to register.
Zoom access link will be sent to your registration email.

Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
2:00pm - 2:05pm Introduction to CABS, Dr. Carrie Wang, President-elect of CABS
2:05pm - 2:45pm Stress Relief and Mental Health in COVID-19 Pandemic. Dr. Lu Ye, Department of Psychiatry, Kaiser Permanente
2:45pm - 3:00pm Q&A

Speaker

Lu Ye, MD
Dr. Lu Ye graduated from medical school in West China university of Medical Sciences. She completed her psychiatry residency and fellowship training in University of Louisville, KY. Dr. Ye has practiced Psychiatry for 20 years, both in USA and China. She provided teaching and clinical supervision for medical students and residents for years. Her research involved pharmacotherapy in depression, anxiety and many other mental health areas. Dr. Ye cares for individuals with a variety of mental health conditions, including depression, anxiety, ADHD, PTSD, bipolar disorder, autism, and psychosis, across acute care, inpatient, and outpatient settings.
Free registration here and is required to obtain Zoom Conference access link, which will be sent to your registration email.Recommend Using PC/Mac to Register. If CANNOT register using an iPhone, click here.
You can also register free at Eventbrite, click here to register.
Special Thanks to Zoom Video Communications and Beijing Well-being Foundation for technical support.

Zoom Conference
On May 9, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled “Stress Relief and Mental Health in COVID-19 Pandemic”. Dr. Lu Ye, a psychiatrist from Kaiser Permanente presented the talk.
Dr. Carrie Wang started the webinar with an introduction of CABS and its efforts combating COVID-19, including a series of scientific webinars and a PPE donation campaign.
Dr. Lu Ye has been practicing mental health therapy and consultation for more than 20 years in both USA and China. Dr. Ye started the talk with methods to cope with anxiety by self-care. She recommended music, dance, read etc, to distract yourself from stress and anxiety. “treat yourself with good food and talk to your friends will also be very helpful”, Dr. Ye said.
Dr.Ye than discussed children home schooling and how to work from home with kids. Setting up daily schedule and routine is very important. For younger children, set up a reward system will help. For older children, let them start the day with an activity they like.
Dr. Ye also recommend constant communication with older family members such as parents and grandparents. “This is very important because they are high risk for COVID-19 and we are there to help”, Dr. Ye said.
Dr. Ye conclude the talk with tips on how to prepare for the reopening. “reopening is inevitable”, Dr. Ye said, “do remind yourself that this is temporary”. Dr. Ye recommended to have a plan before the reopen and prepare for what to do under different situation. Seeking help from friends and co-workers is also important.After the presentation, Dr. Ye answered questions from moderator Dr. Carrie Wang and also from audiences.
The event lasted one hours and ten minutes The webinar attracted more than 100 audiences from around the globe.
We would like to thank Dr. Lu Ye for her time and efforts for this webinar. We also want to thank Jenen Tan, Hesong Han, Xu Chen, Carrie Wang, for organizing the logistics of the event and thanks to Zoom and Beijing Well-being Foundation for providing technology support for this webinar. To learn more about CABS’ effort in the COVID-19 response, please visit our website www.cabsweb.org. A replay of the webinar recording will be available on this site.
Online
is
CABS-GLF Webinar
COVID-19 Pandemic Requires Global Collaborations
US Pacific Time: May 2, 2020, 5:00pm-7:00pm,
China Beijing Time: May 3, 2020, 8:00am-10:00am

A Dialogue Among Frontline Physicians Battling COVID-19 in China and the United States
- How to prevent mild patients from transitioning into severe patients
- Current COVID-19 epidemiology features
- First-hand clinical experiences from China and US hospitals
- A story of converting an academic lab into a COVID-19 test lab
 Please use the following Zoom link to access the webinar:
Please use the following Zoom link to access the webinar:
https://zoom.us/s/98875114106
Or
dial US :+1 646 558 8656 or +1 669 900 9128 or +1 253 215 8782 or +1 301 715 8592 or +1 312 626 6799 or +1 346 248 7799
Webinar ID: 988 7511 4106
Free Registration by clicking here or "Register" button at bottomOr
Free Registration at Eventbrite, click here to register.

Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
5:00pm - 5:05pm Introduction to CABS, Dr. Carrie Wang, President-elect of CABS
5:05pm - 5:10pm Introduction to GLF, Dr. Weixing Chen, President of Gracious Life Foundation
5:10pm - 5:30pm COVID-19 Challenge: Diagnosis and Treatment in Clinic Practice. Dr. Fengming Luo, Professor of Respiratory Therapy, West China Medical School
5:30pm - 5:45pm Epidemiology of COVID-19 Pandemic and Potential Global Collaboration. Dr. Zuo-Feng Zhang, Professor of Epidemiology, UCLA
5:45pm - 6:00pm COVID-19: Challenges in a US ICU. Dr. Michael Bernstein, Pulmonologist, Stamford Hospital Connecticut
6:00pm - 6:15pm How to Setup a Pop-up SARS-CoV-2 Diagnostic Testing Facility at a University without a Medical School in Just Three Weeks? Dr. Lea Witkowsky, Policy Analyst, UC Berkeley
6:15pm - 7:00pm Panel Discussion.
Moderator: Dr. Hua Jiang, Infectious Disease Specialist, Methodist Hospital of Southern California
Panelists: Dr. Fengming Luo, Dr. Zuo-Feng Zhang and Dr. Michael Bernstein, Dr. Lea Witkowsky.

Speaker

Fengming Luo, MD, PhD
Dr. Fengming Luo is currently the Professor and Dean of Department of Respiratory Therapy, West China Medical School, Sichuan University. He is also the Vice Director, Department of Respiratory and Critical Care Medicine at West China Hospital. Dr. Fengming Luo has been the team lead of medical response team in Wuhan and responsible for 1 ICU and 2 Wards with 98 hospital beds and treated over 300 COVID-19 patients.Dr. Fengming Luo obtained his MD from Chengdu University of Traditional Chinese Medicine, Chengdu, China, MS degree in Medicine from Fujian University of Traditional Chinese Medicine and PhD in Medicine from West China Medical Center, Sichuan University, Chengdu. From 2007 to 2008, he was a Visiting scholar in the Lab of Pulmonary Biolog at Cincinnati Children’s Hospital Medical Center, OH, US.
Zuo-Feng Zhang, MD, PhD
Dr. Zuo-Feng Zhang is a Senior Professor of Epidemiology and Medicine and Associate Dean for Research of the UCLA Fielding School of Public Health. He is also Co-Director of the UCLA AlperCenter for Environment Genomics, and Director of Cancer Molecular Epidemiology Training Program. Dr. Zhang had been a Fellow of the American College of Epidemiology (ACE) and Member of the Board of Directors of the American College of Epidemiology. He was a World Health Organization (WHO) Consultant for National Non-communicable Disease Prevention and Controls in China. He studied preventive medicine and epidemiology in Shanghai Medical University from 1978 to 1987
Hua Jiang, MD
Dr. Hua Jiang is a board-certified Infectious Diseases specialist at the Methodist Hospital of Southern California in Arcadia and Emanate Health (formerly Citrus Valley Medical Center) in Covina, California. A native of Shanghai, he graduated from Shanghai First Medical College (currently Shanghai Medical School, FudanUniversity) in 1983, as one of the top ten graduates in a class of more than 350 students. After graduation, he went on to specialize in Infectious Diseases in Hua Shan Hospital, a premier hospital in China, especially known for its Infectious Diseases program. In 1986, Dr. Jiang came to Harvard Medical School to work at the WHO Collaborative Research Laboratory for Bacterial Resistance Surveillance as a research scholar, studying mechanisms of bacterial resistance to antibiotics. He is current the Chair of Infection Control, Methodist Hospital of Southern California.
Michael Bernstein, MD
Dr. Bernstein is a Co-Clinical Director for the Lung Cancer Screening and Lung Nodule Program at Stamford Hospital with training at Duke University and Mount Sinai Medical Center in New York City. He is also an Assistant Clinical Professor of Medicine in Stamford for Columbia University College of Physicians and Surgeons. Dr. Bernstein has a particular interest and board-certification in interventional pulmonology, a subspecialty within pulmonary medicine focused on bronchoscopy, lung cancer, pleural diseases and other procedures for the lung. Dr. Bernstein is the Associate Director for Pulmonary and Critical Care at Stamford Hospital. A pulmonologist who treats severe Covid patients in Connecticut, where is badly hit by this Covid as part of areas near New York.
Lea Witkowsky, PhD
Dr. Lea Witkowsky is a Policy Analyst at the Innovative Genomics Institute (IGI), a non-profit research partnership between UC Berkeley and UC San Francisco, dedicated to improving and applying genome engineering to solve major world problems. Lea received her PhD from UC Berkeley in 2016, where she studied mechanisms of gene regulation and CRISPR-based gene editing in human cells. She joined the IGI in 2017 to work on engagement, science policy, and social impacts, but has recently pivoted to focus on the IGI’s COVID-19 response. In just three weeks a team at IGI transitioned an academic research lab at a university without a medical center into a CLIA-certified, SARS-CoV-2 clinical diagnostic facility. Lea co-led the effort to document the process in a detailed blueprint for other universities to follow and has led the establishment of policies for the allocation of our testing capacity. When not working on COVID-19, Lea runs an ethics and regulation working group that brings together life scientists, social scientists, lawyers, and ethicists. She aims to inspire and facilitate scientifically informed ethical discussions about genome editing through communications with regulators and policymakers and by convening international events that feature diverse perspectives.
Free registration here and is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.
Recommend Using PC/Mac to Register. If CANNOT register using an iPhone, click here.
You can also register free at Eventbrite, click here to register.
Special Thanks to Zoom Video Communications and Beijing Well-being Foundation for technical support.

Zoom Conference
is
Summary and Replay of CABS-GLF Webinar
COVID-19 Pandemic Requires Global Collaborations

Watch a Replay Recording of this Webinar

On May 2, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted a webinar entitled “COVID-19 Pandemic Requires Global Collaborations”. The event was co-organized by the Gracious Life Foundation (GLF). Five frontline physicians and scientists who are combating COVID-19 gave a series of mini-talks followed by a panel discussion on current COVID-19 pandemic.The webinar attracted more than 700 healthcare and life sciences professionals from around the globe, including many frontline physicians and nurses who are fighting the disease themselves (see below audience map).

Dr. Carrie Wang started the webinar with an introduction of CABS and its efforts combating COVID-19, including a series of scientific webinars and a PPE donation campaign. Dr. Weixing Chen followed with an introduction of Gracious Life Foundation.

Dr. Fengming Luo, Professor of Respiratory Therapy at West China Medical School, gave the first mini-talk on COVID-19 Challenge: Diagnosis and Treatment in Clinic Practice. Dr. Luo was the leader of the West China Medical School’s medical response team in Wuhan. He was responsible for one ICU and two wards comprising 98 hospital beds and he has treated over 300 COVID-19 patients. Dr. Luo discussed the diagnostic and treatment of COVID-19 from his Wuhan experience. The key for accurate diagnostic of COVID-19 is combining clinic history and symptoms, nucleic acid test, antibody test and imaging all together because each single method has limitations. Also, preventing the disease from worsening and providing respiratory support is very important for COVID-19 treatment. “To Comfort Always, means we should treat patients, not only with medicine, but also with psychology”, Dr. Luo concluded his talk.
Dr. Zuo-Feng Zhang, Professor of Epidemiology from UCLA, gave the second mini-talk on Epidemiology of COVID-19 Pandemic and Potential Global Collaboration. Dr. Zhang gave a brief history of COVID-19 epidemiology from Wuhan to the rest of the world. He observed that Asian countries showed a lower mortalityper million population than other countries. The underlying reasons for this finding still need to be elucidated. “A better understanding of the COVID-19 epidemiology and control of COVID-19 pandemic require global collaboration”, Dr. Zhang concluded his talk.
Dr. Michael Bernstein, a Pulmonary/Critical Care Physician from Stamford Hospital Connecticut gave the third mini-talk on COVID-19: Lessons from ICU Expansions. Dr. Bernstein discussed six challenges in expanding ICU to cover COVID-19 patients in Stamford hospital. These include physical location of ICU, medical staff, non-medical staff, equipment/proning, medications, and reporting/communications. “The most critical part and also the biggest challenge is to build a medical team that can work together efficiently during the pandemic situation”, Dr. Bernstein concluded his talk.
Dr. Lea Witkowsky, a Policy Analyst from UC Berkeley, gave the last mini-talk talk on How to Setup a Pop-up SARS-CoV-2 Diagnostic Testing Facility at a University without a Medical School in Just Three Weeks? Dr. Witkowsky presented a few key steps to convert an academic lab to a certified COVID-19 testing lab, including regulation compliance, personnel to run the test, scaling up the test, RT-PCR primers and probes, sample preparation, and the target testing population. For example, they came up with their own design of sample collection tubes prefilled with DNA/RNA shield solution to inactivate the virus before delivering the sample to the testing lab. “It is an amazing team effort to come up with a certified testing lab within a month”, Dr. Lea Witkowsky said, “thanks to all the collaborators and volunteers”.

After the mini-talks, Dr. Hua Jiang, an Infectious Disease Specialist from Methodist Hospital of Southern California, moderated a panel discussion with all four speakers. Various questions on the diagnostics, treatment and epidemiology of COVID-19 were discussed.
The event lasted two hours and forty minutes and there were still close to 290 members in the audience at the end, showing the great interest generated by the discussion. The webinar concluded with a video showing the donation activity from both CABS and GLF to various US hospitals. More than 100 audiences stayed until the end of the video.
We would like to thank Dr. Fengming Luo, Dr. Zuo-Feng Zhang, Dr. Michael Bernstein, Dr. Lea Witkowsky and Dr. Hua Jiang for their time and efforts for this webinar. We also want to thank Jenen Tan, Guanghui Han, Xu Chen, Carrie Wang, Weixing Chen for organizing the logistics of the event and thanks to Zoom and Beijing Well-being Foundation for providing technology support for this webinar. To learn more about CABS’ effort in the COVID-19 response, please visit our website www.cabsweb.org.
Fengming Luo, MD, PhD
Dr. Fengming Luo is currently the Professor and Dean of Department of Respiratory Therapy, West China Medical School, Sichuan University. He is also the Vice Director, Department of Respiratory and Critical Care Medicine at West China Hospital. Dr. Fengming Luo has been the team lead of medical response team in Wuhan and responsible for 1 ICU and 2 Wards with 98 hospital beds and treated over 300 COVID-19 patients.Dr. Fengming Luo obtained his MD from Chengdu University of Traditional Chinese Medicine, Chengdu, China, MS degree in Medicine from Fujian University of Traditional Chinese Medicine and PhD in Medicine from West China Medical Center, Sichuan University, Chengdu. From 2007 to 2008, he was a Visiting scholar in the Lab of Pulmonary Biolog at Cincinnati Children’s Hospital Medical Center, OH, US.
Zuo-Feng Zhang, MD, PhD
Dr. Zuo-Feng Zhang is a Senior Professor of Epidemiology and Medicine and Associate Dean for Research of the UCLA Fielding School of Public Health. He is also Co-Director of the UCLA AlperCenter for Environment Genomics, and Director of Cancer Molecular Epidemiology Training Program. Dr. Zhang had been a Fellow of the American College of Epidemiology (ACE) and Member of the Board of Directors of the American College of Epidemiology. He was a World Health Organization (WHO) Consultant for National Non-communicable Disease Prevention and Controls in China. He studied preventive medicine and epidemiology in Shanghai Medical University from 1978 to 1987
Hua Jiang, MD
Dr. Hua Jiang is a board-certified Infectious Diseases specialist at the Methodist Hospital of Southern California in Arcadia and Emanate Health (formerly Citrus Valley Medical Center) in Covina, California. A native of Shanghai, he graduated from Shanghai First Medical College (currently Shanghai Medical School, FudanUniversity) in 1983, as one of the top ten graduates in a class of more than 350 students. After graduation, he went on to specialize in Infectious Diseases in Hua Shan Hospital, a premier hospital in China, especially known for its Infectious Diseases program. In 1986, Dr. Jiang came to Harvard Medical School to work at the WHO Collaborative Research Laboratory for Bacterial Resistance Surveillance as a research scholar, studying mechanisms of bacterial resistance to antibiotics. He is current the Chair of Infection Control, Methodist Hospital of Southern California.
Michael Bernstein, MD
Dr. Bernstein is a Co-Clinical Director for the Lung Cancer Screening and Lung Nodule Program at Stamford Hospital with training at Duke University and Mount Sinai Medical Center in New York City. He is also an Assistant Clinical Professor of Medicine in Stamford for Columbia University College of Physicians and Surgeons. Dr. Bernstein has a particular interest and board-certification in interventional pulmonology, a subspecialty within pulmonary medicine focused on bronchoscopy, lung cancer, pleural diseases and other procedures for the lung. Dr. Bernstein is the Associate Director for Pulmonary and Critical Care at Stamford Hospital. A pulmonologist who treats severe Covid patients in Connecticut, where is badly hit by this Covid as part of areas near New York.
Lea Witkowsky, PhD
Dr. Lea Witkowsky is a Policy Analyst at the Innovative Genomics Institute (IGI), a non-profit research partnership between UC Berkeley and UC San Francisco, dedicated to improving and applying genome engineering to solve major world problems. Lea received her PhD from UC Berkeley in 2016, where she studied mechanisms of gene regulation and CRISPR-based gene editing in human cells. She joined the IGI in 2017 to work on engagement, science policy, and social impacts, but has recently pivoted to focus on the IGI’s COVID-19 response. In just three weeks a team at IGI transitioned an academic research lab at a university without a medical center into a CLIA-certified, SARS-CoV-2 clinical diagnostic facility. Lea co-led the effort to document the process in a detailed blueprint for other universities to follow and has led the establishment of policies for the allocation of our testing capacity. When not working on COVID-19, Lea runs an ethics and regulation working group that brings together life scientists, social scientists, lawyers, and ethicists. She aims to inspire and facilitate scientifically informed ethical discussions about genome editing through communications with regulators and policymakers and by convening international events that feature diverse perspectives.
Special Thanks to Zoom Video Communications and Beijing Well-being Foundation for technical support.
Zoom Conference
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Disclaimer: the following summary of CABS webinar contains discussion on the potential use of certain drugs (such as hydroxychloroquine and others) for COVID-19. None of the drugs has been approved to treat COVID-19 at the moment of the webinar and the writing of this summary (US FDA issued EUA use of hydroxychloroquine). Please DO NOT use those drugs for COVID-19 without an official prescription from a licensed physician or doctor. CABS and Professor Wenhong Zhang will not hold liability for any consequence of using the drugs discussed in the webinar and this summary.
 On March 24, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted the webinar “COVID-19 clinical treatment and research experiences, with Dr. Wenhong Zhang”. The event was co-organized by the Society of Chinese American Physician Entrepreneurs (SCAPE) and the Association of Fudan Alumni in Healthcare. Dr. Zhang is the Director of Department of Infectious Diseases, Huashan Hospital, Fudan University, and the leading physician in the COVID-19 expert team in Shanghai. He presented valuable insights in controlling the COVID-19 outbreak and treating COVID-19 patients. The webinar attracted >10,000 healthcare and life sciences professionals from around the globe, including many frontline physicians and nurses who are fighting the disease in the U.S. and Europe (see the audience location map below).
On March 24, 2020, the Chinese American Biopharmaceutical Society (CABS) successfully hosted the webinar “COVID-19 clinical treatment and research experiences, with Dr. Wenhong Zhang”. The event was co-organized by the Society of Chinese American Physician Entrepreneurs (SCAPE) and the Association of Fudan Alumni in Healthcare. Dr. Zhang is the Director of Department of Infectious Diseases, Huashan Hospital, Fudan University, and the leading physician in the COVID-19 expert team in Shanghai. He presented valuable insights in controlling the COVID-19 outbreak and treating COVID-19 patients. The webinar attracted >10,000 healthcare and life sciences professionals from around the globe, including many frontline physicians and nurses who are fighting the disease in the U.S. and Europe (see the audience location map below).
Dr. Zhang first highlighted the success of COVID-19 infection control in Shanghai. One model had predicted Shanghai would see 800,000 COVID-19 cases, but as of now only around 400 have emerged. The key, as Dr. Zhang pointed out, was early control of local transmission. The first case in Shanghai, like in US, was identified on Jan 21, 2020. At that time, Wuhan’s lockdown suppressed most of the intercity traffic from Hubei provinces to Shanghai. Shanghai, at the meantime, immediately took aggressive measures to “slow down” the city, including cancelling all gathering activities, suggesting all citizens to stay at home unless necessary activities, and extending the Spring Festival holiday to 14 days, thus allowing Shanghai’s social distance to overlap the length of a 14-day incubation period if anyone really had a possible infection through encountering an imported case. At the same time, groceries, supermarkets, hospitals, banks and other life necessities facilities remained open for normal life. These extraordinary early efforts effectively prevented the spread of the virus.
In Shanghai, a network of 117 fever clinics and an additional 182 fever sentinel hospitals were positioned to collect all samples from suspicious cases meeting one epidemiology criterion and one relevant clinical symptom. All suspected patients met with the criterion could be tested freely and were admitted to the hospital quarantine ward to receive further evaluation by the CDC staff. Samples were then sent to CDC for diagnosis and confirmed COVID-19 patients were immediately transferred to designated hospitals.
In regard to the diagnosis, Dr. Zhang shared his knowledge on three aspects: co-infection, false negative rate of RT-PCR, and value of CT scan. First, co-infection was not uncommon: 11 out 20 patients contracted both COVID-19 and other pathogens. Secondly, false negative rate was relatively high (~30% of total samples), especially for the first-time PCR test. The false negative rate dropped another 50% (to 15% of total samples) for the second-time PCR test. The reason for RT-PCR false negativity might be due to the quality of the kit, the laboratory technique, the sampling technique and the sampling site. If a patient still tested negative after two PCRs but showed clinical symptoms, next generation sequencing (NGS) would follow and could confirm all diagnoses. Lastly, although chest CT scan could be used to detect COVID-19, the CT scan could share the similar resemblance in non-COVID-19 pneumonia patients. Therefore, COVID-19 diagnosis by CT scan alone was not adequate; PCR tests represented the first line diagnosis for patients.
To summarize, suspected cases were enrolled and diagnosed at Huashan Hospital using the following protocol (Figure 5 of this publication https://www.medrxiv.org/content/10.1101/2020.02.13.20022673v1.full.pdf). To rule out false negative cases, suspected cases had to pass two COVID-19 PCR tests. If cases tested negative twice in a roll but were highly suspicious by clinical symptoms or if the patients were in severe conditions, a third test would be performed either by repeating the PCR test, or performing mNGS, or arranging the test by a third party.
Timely and extensive testing of the suspected population is crucial in the prevention and control of the COVID-19. The timely diagnosis protocol and timely quarantine measure in Shanghai effectively stopped the circulation of COVID-19 in the city, without causing overwhelming impact on the normal citizen’s life. Dr. Zhang’s team also found that the incubation time, as measured as days from suspected contact to symptom onsets is about 6.4 days (mean); and the mean onset-hospital admission interval was 5.5 days.
In regard to treatment options for COVID-19, Dr. Zhang indicated that no effective antiviral treatment had been identified to this date. Several drugs, including arbitol, lopinavir/ritonavir, hydroxychloroquine sulfate, and remdesivir are currently invested in clinical trial. Shanghai was the first city to use hydroxychloroquine in the treatment, but evidence for its clinical efficacy was not significant; nevertheless, toxicity of hydroxychloroquine seems low and doctors can consider it as treatment option. Sufficient respiratory support, especially oxygen therapy, made a key difference in the survival of patients. A paper published by Dr. Yuanlin Song reported the invasive mechanical ventilation support rate and mortality rate in Wuhan, and when we compared that data with that of Shanghai, the result would suggest that a higher ventilation support rate led to a significantly lower mortality.
Recent studies showed that organ and coagulation dysfunction (e.g. higher lactate dehydrogenase and D-dimer) were crucial factors for the progression from acute respiratory distress syndrome (ARDS) to death, so anticoagulation at the early stage could help reduce microthrombus. Although no strong clinical evidence of anticoagulant or corticosteroids had been observed, Dr. Zhang found that the use of corticosteroids could sometimes slow the progression of disease and reduce the severity in patients with ARDS. Regardless, timely life support for critically ill patients, such as cannula or mask oxygen inhalation, high-flow nasal cannula oxygen therapy, invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO), is important. Organ support, hemodynamic stability, nutritional support, analgesia and sedation should also be maintained. The overall mortality rate of COVID-19 is around 3-4% (<1% in Shanghai), but there is only a short time window for intervention during the treatment.
Finally, protecting medical personnel is crucial. None of the 400 medical personnel from Shanghai who went to support Wuhan was infected. To achieve this, a standardized process needs to be established to allow access to appropriate personal protective equipment (PPE). These include medical protective masks, isolation gowns, goggles or visors, double-layer gloves, double-layer shoe covers, etc. Positive pressure breathing masks are recommended for medical personnel. Disinfection and isolation are also important for protection. Using hydrogen peroxide to disinfect the environment at least once every 4 hours and at the end of procedures is recommended.
At the end of the webinar, Dr. Zhang and his team answered dozens of questions, pre-submitted by the audience, related to COVID-19 diagnosis, disease progression, clinical treatment, discharge and follow-up, as well as protection for medical personnel (see below for details of the Q&A during the webinar. CABS will follow up another email or posting on Dr. Wenhong Zhang’s answer to questions from the audiences after the webinar). A publication from Dr. Wenhong Zhang lab is here and contains data for the webinar (https://www.medrxiv.org/content/10.1101/2020.02.13.20022673v1.full.pdf).
We thank Dr. Jingwen Ai for answering some of these questions and Mr. Yifan Wang for assisting with the logistics. We really appreciate the valuable insights from Dr. Zhang and his team, and we will work in solidarity to fight this global pandemic.
Q1: What is the time window between infection and testing positive?
A: PCR test will usually turn positive under 2-3 days after the infection. Serological test i.e. antibody- based test (IgM) would mostly turn positive in 8-10 days after the disease onset. Viral load is the highest during the first week of disease onset and would decrease later.
Q2: For the general public, what are the criteria to request a SARS-CoV-2 test? For healthcare workers?
A: In Shanghai, people who meet at least one of the epidemiological criteria and one of the clinical criteria should request a test. Originally, only people who returned from Wuhan and Hubei province would meet the epidemiological criteria. Now people with travel history to any countries/regions with local transmission cases meet the epidemiological criteria. People should also request a test if they have close contact with confirmed patients or if clustered cases were observed around them. Clinical criteria include symptoms such as coughing, fever and diarrhea. We try to test every suspected case in Shanghai and this is the key to closely monitoring the epidemic trend. All tests are provided free of charge.
Q3: What are the pros and cons of PCR-based test Vs antibody-based test?
A: PCR test is the gold standard. We are still gaining more experiences concerning antibody-based tests in Shanghai. Currently, antibody-based test can serve as a complementary test to the PCR test. It might work better after 8-10 days of disease onset because the viral load might be too low to be detected by PCR test (false negative) in the later stage. Antibody tests could also be a useful tool to understand the epidemic trend for a large population.
Q4: How to prevent viral RNA degradation during sample processing?
A: The sample should be processed as soon as possible (usually within 4-6 hours), and if not, sample should be stored at -20C/-80C.
Q5: For people who are self-quarantined for 14 days at home, should they be tested? If yes, when and how frequent?
A: If no symptom was observed after quarantine, no test is needed.
Q6: What is the risk of getting infected for pregnant women and infants compared to other demographic groups? If infected, what are the treatment options?
A: Not many cases of pregnant women and infants were reported in Shanghai. Only 50-60 children were treated and they all have mild symptoms. No anti-viral treatment was used on children in Shanghai. No severe cases for pregnant women, infant and children observed in Shanghai.
Q7: From mild to severe symptoms, how long will it take?
A: Usually 8-9 days.
Q8: Why people with underlying medical conditions are at high risk? Young age but with underlying conditions are also at high risk?
A: Young people with underlying medical conditions are also at high risk. People with heart disease have the highest risk.
Q9: At large population level, do you observe the attenuation of the infectivity or pathogenicity of SARS-CoV-2 over time?
A: Not observed so far. The number of severe cases has decreased but there’s probably no attenuation of the infectivity of the virus.
Q10: Ibuprofen VS acetaminophen, which one is better to treat fever at home for mild COVID-19 patient?
A: Acetaminophen is recommended because of fewer side effects.
Q11: Literature reported that ARB inhibitor like Losartan can increase cell surface ACE2 expression. Do you observe people who are taking ARBi showing a higher chance of SARS-CoV-2 infection? How about use Losartan to reduce lung inflammation in COVID-19?
A: We did not observe people who are taking ARBi showing a higher chance of SARS-CoV-2 infection. ARBi losartan has not shown effect in reducing lung inflammation in SARS-Cov-2 cases.
Q12: What is the percentage of recovered patients showing detectable neutralizing antibodies against SARS-CoV-2? What type of antibodies? What is the efficacy against COVID-19?
A: Antibodies were detected in all recovered patients. It’s still hard to say if they have efficacy or not. It also depends on the type of antibody produced.
Q13: Will upcoming summer slowdown global pandemic of COVID-19?
A: Summer might help a little bit, as the activity of the virus has shown to decrease with the temperature rising. However, this phenomenon was mostly observed in the laboratory, and the temperature would need to be as high as around 60℃ to kill the virus. Therefore, the summer might slow down the transmission in the northern hemisphere to some extent, but we still need to remember the risks of epidemics in the southern hemisphere will not diminish by that time. COVID-19 might not be eliminated this year. It might reappear in the winter. The coming 2-3 months is critical for US to take actions to slowdown the spread of the disease.
Q14: Can hydroxychloroquine help to prevent COVID-19? Dose and side effects?
A: Data currently available is controversial. However, it’s probably OK for medical personnel and family members to take hydroxychloroquine under strong cautions to prevent COVID-19, as long as no significant side effect is observed.
Q16: How to use hydroxychloroquine for COVID-19 treatment? Dose and frequency?
A: The dosage we used was day 1: 400mg b.i.d., day 2 and after: 400mg q.d. Only monotherapy has been used. We are worried about liver injury caused by the combination therapy with azithromycin, and that’s why we have not tried the combination of hydroxychloroquine and azithromycin. We have not tried the hydroxychloroquine and zinc combination either.
Q18: How important it is to cover head/neck/shoes when treating COVID patients?
A: Covering the head is the most important.
Q19: How often are HCWs infected from the virus?
A: Only HCW in Wuhan without proper PPE at the beginning of the outbreak were infected. None of the HCW in Shanghai or HCW sent to Wuhan from Shanghai with proper PPE was infected.
Q20: Is regular surgical mask adequate for HCWs to prevent COVID-19 under mild patient and severe patient?
A: No. N95 is required. PPE is very important.
Q21: Are you routinely testing healthcare workers who are exposed to COVID+ patients?
A: In Huashan hospital we test HCWs. However, it’s probably not necessary to do the test if people wear proper PPE and no symptom is observed.
Q22: Can COVID-19 be transmitted by air droplet/airborne, in closed spaces, such as elevator, waiting room?
A: Air droplet would impose a risk to people without proper PPE (masks). However, in our hospital no patient was infected via air droplet in the elevator or fever clinics. Only close contact with an infected person would transmit the disease.
Q23: At US, healthcare workers go home after work. What procedure need to be done to prevent spreading the virus to family members?
A: Healthcare workers at Shanghai Public Health Clinical Center would stay at the hospital. Those who work at the Shanghai Children’s Hospital do go home every day. They wear proper PPE, take bath and wash face and nose frequently. In addition, they do not have dinner together with their family members.
Q24: For hospital departments that do not directly interact with COVID-19 patients, can healthcare workers use reduced PPE?
A: No.
Q25: For healthcare workers that interact with COVID-19 patients, what need to be done before eating and drinking?
A: Proper PPE is required, and frequent cleaning of face and nose is recommended.
Q26: What is the procedure to discharge COVID-19 recovered patients? Do they need to self-quarantine at home for 14-days?Is there a possibility of viral rebound?
A: The patient can be discharged if tested negative by PCR test twice. After discharge, we require the patient to be quarantined at home for 14 days.
Q27: If a patient survived severe COVID-19, will his/her lung surfer long term injury? Fibrosis?
A: Unlike SARS patients, most severe COVID-19 patients fully recovered in less than two weeks. They are all in good conditions with no or minimal fibrosis.
To learn more about CABS’ effort in COVID-19 response, please visit our website www.cabsweb.org.
Please contact: info@cabsweb.org for any question related to COVID-19.

Online
CABS Bulletin Board for COVID-19 Supply Requests
As the leading life sciences professional organization in the Bay Area, the Chinese American Biopharmaceutical Society (CABS) has received requests from local hospitals and testing centers for laboratory/medical supplies such as instruments, reagents, and testing kits. Therefore, we wish to create this website to serve as a bulletin board for such requests. We would like to ask our CABS community to please respond to or forward the following survey if your know of any organization in need of such supplies or can provide these supplies.
Please note that this bulletin board is not designated for personal protection equipments (PPEs) such as facial masks or protection covers. We recognize that there is also a severe shortage for PPEs and we encourage interested parties to leverage other resources such as www.getusppe.org, www.mask-match.com, www.donateppe.org.
Please click this link if your organization have needs for laboratory/medical supplies.
The information you submitted will be posted on the CABS website and distributed to the public via the CABS network. Please check the following table for supply requests submitted previously. We strive to update this bulletin board as soon as we receive the requests, but we cannot guarantee the timeliness of these requests as the situation can change rapidly. We encourage to contact the organizations in need directly.
For collaborations and partnerships or if you have any suggestions or comments about this bulletin board, please email public_communication@cabsweb.org.
Online
CABS Webinar
COVID-19 Clinical Treatment and Research Experiences from China, with Dr. Wenhong Zhang
US Pacific Time: March 24, 2020, 5pm-6pm
US Eastern Time: March 24, 2020, 8pm-9pm
China Beijing Time: March 25, 2020, 8am-9am
The webinar is delivered through Zoom Conference
Agenda (time is based on US Pacific Time)
5:00-5:05 PM PST: Introduction to CABS. Yang Tian, President of CABS [English]
5:05-5:35 PM PST: COVID-19 clinical treatment and research experiences from China. Dr. Wenhong Zhang. Director of Department of Infectious Diseases, Huashan Hospital, Fudan University [English]
5:35-6:00 PM PST: Q&A [Chinese]
Free registration:
1. Register using a PC or laptop or Android Phone, click "Registration" button at bottom of page or register at eventbrite.
2. Register using an iPhone or iPAD, at eventbrite. (iOS user please use Eventbrite link to register)
Registration is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.
Speaker Introduction
Professor Zhang Wenhong is currently the Director of the Department of Infectious Diseases of Huashan Hospital, Fudan University, the leader of Shanghai Anti-COVID-19 clinical expert team. He is also the secretary-general of the Chinese Society of Infectious Disease, and the vice-chairman of the Chinese Association of Internal Medicine. Professor Zhang graduated from the Medical Department of Shanghai Medical University. He has worked as a visiting scholar and postdoctoral fellow at the Department of Microbiology at the University of Hong Kong, Harvard Medical School, and the Chicago State University. He has in-depth experiences in the diagnosis and treatment of various emerging infectious diseases. During the fight against COVID-19, Professor Zhang led the Shanghai expert team to publish the Shanghai anti-COVID-19 clinical treatment plan and shared it with international colleagues. Professor Zhang is also the Associate Editor of Emerging Microbes and Infections, International Journal of Tuberculosis and Lung Diseases.

Webinar Abstract
After the World Health Organization (WHO) declared the COVID-19 pandemic, Europe has become an epicenter of the outbreak. Recently, the number of confirmed cases of COVID-19 in the United States has also increased rapidly, and various state and local governments have adopted control measures such as shelter-in-place. During the COVID-19 outbreak in China, Professor Zhang Wenhong served as the leader of the Shanghai Anti-COVID-19 clinical expert team. He treats patients infected with COVID-19 at their bedside and is also involved in clinical research. In this webinar, Professor Zhang will share with the audience the prevention and control strategies used in Shanghai and his treatment and research experiences.
This webinar is hosted by the Chinese American BioPharmaceutical Society (CABS) and supported by the Society of Chinese American Physician Entrepreneur (SCAPE) and Association of Fudan Alumni in Healthcare.


Free registration:
1. Register using a PC or laptop or Android Phone, click "Registration" button at bottom of page or register at eventbrite.
2. Register using an iPhone or iPAD, at eventbrite. (iOS user please use Eventbrite link to register)
Registration is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.
Special thanks to Zoom Conference and Beijing Welfare Foundation for their technical support and free access to Zoom.


Zoom Conference
Our online donation campaign is now closed. CABS will use the remaining time to distribute the donation to hospitals and organizations that need help.
Update 1 - 03/27/2020 CABS donated 1,500 surgical masks to San Mateo Medical Center. ($amount=$712.5)
Update 2 - 03/28/2020 CABS donated 400 KN95 masks to New York City Health - Queens Hospital Center ($amount=$1040)
Update 3 - 03/31/2020 CABS donated 100 protective suits to New York City Health - Queens Hospital Center ($amount = $774.9)
Update 4 - 04/06/2020 CABS donated 4000 surgical masks to 3 hospitals ($amount = 1466)
Update 5 - 04/19/2020 CABS donated 237 Coverall Protective Gowns to 3 New York Hospitals ($amount = $4664)
Update 6 - 04/22/2020 CABS sourced 3060 N95 masks; 2040 of those were donated to 7 hospitals; remaining 1020 are in transit ($amount = $15,000)
Update 7 - 04/22/2020 CABS donated 2000 face shields to 10 hospitals around the US
Update 8 - 04/23/2020 CABS donated 1000 Protective Gowns to 10 hospitals around the US

From March 22 to April 4, 2020, total cash donation received:
| Disclosed | Non-disclosed | transaction failed | Total | |
| # of donors | 58 | 56 | 12 | 126 |
| Total $ CABS received | 18260 | 12400 | 0 | 30,660 |
From March 22 to present, total goods donation received:
| Date | Donor | Goods donate to CABS | QTY | $value |
| 3/27/2020 | Jennifer Wang | Yide Disposable masks | 90 | 50 |
| 3/31/2020 | S & Z Trading LLC | Lakeland Coverall | 100 | 496.6 |
| 3/31/2020 | Yinshi Liu | N95 Mask | 100 | 300 |
| 04/06/2020 | Kaining Guo | Surgical Masks | 4000 | 1466 |

Update 1 - 03/27/2020
CABS donated 1,500 surgical masks to San Mateo Medical Center.

Thanks to all CABS donors for your cash donation; to Mei Zhang for providing source of surgical masks; to Carrie Wang, Yan Wang, Xu Chen and all other CABS volunteers for logistic operations!

Update 2 - 03/29/2020
CABS donated 400 KN95 masks to New York City Health and Hospitals - Queens Hospital Center

Thanks to all CABS cash donors; to Dr. Yuan Qin (Beijing) for source of N95 masks; to Carrie Wang, Xu Chen and other CABS volunteers for logistic operations

Update 3 - CABS donated 100 protective suits to New York City Health - Queens Hospital Center
Thanks to Mei Zhang for donating protective suits and masks to CABS !!! to Carrie Wang, Jeff, Xu Chen and other CABS volunteers for logistics!!!

Update 4 (04/06/2020) - CABS donated 4000 surgical masks to 3 hospitals
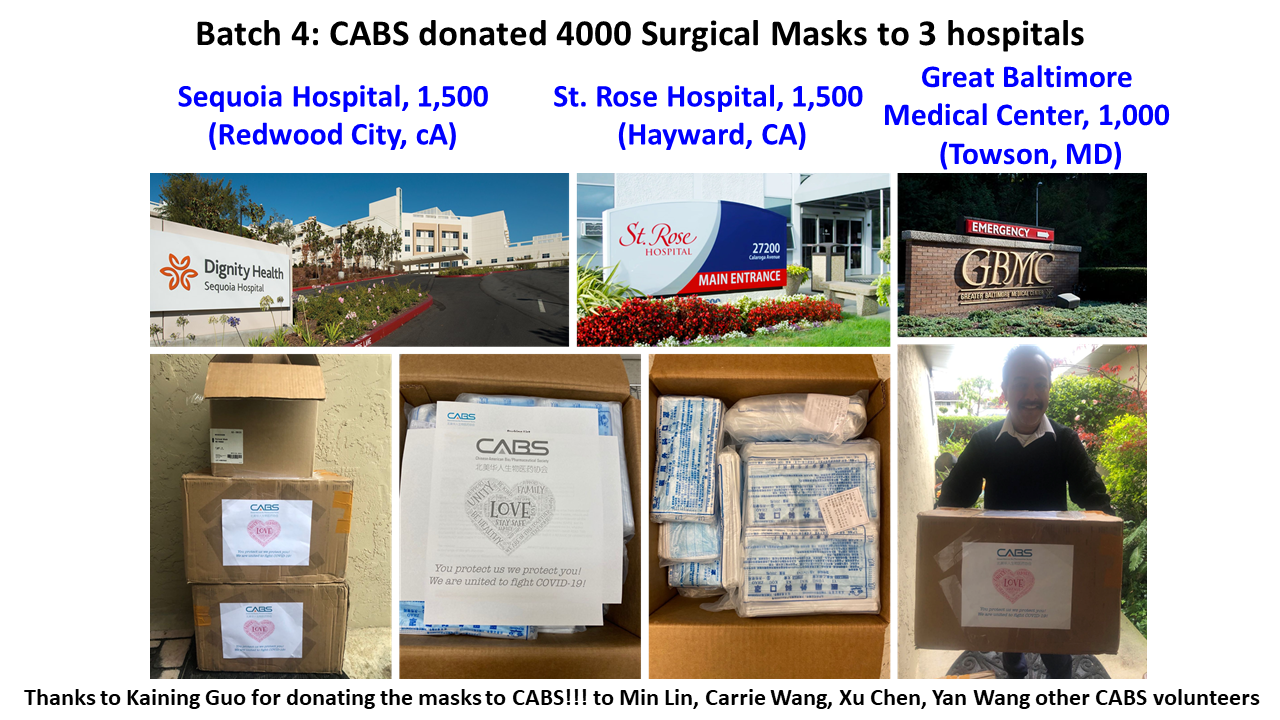
Thanks to Kaining Guo for donating masks to CABS!!! thanks CABS volunteers Min Lin, Carrie Wang, Xu Chen and Yan Wang !!!

Update 5 - 04/19/2020 CABS donated 237 Coverall Protective Gowns to 3 New York Hospitals

Update 6 - 04/22/2020 CABS sourced 3060 N95 masks; 1700 of those were donated to 6 hospitals; remaining 1360 are in transit ($amount = $15,000)
Special thanks to Weixing Chen from GLF for help sourcing N95 masks and handling shipping logistics.

Update 7 - 04/22/2020 CABS donated 2000 face shields to 10 hospitals around the US
Special thanks to Weixing Chen from GLF for help sourcing N95 masks and handling shipping logistics.

Update 8 - 04/23/2020 CABS donated 1000 Protective Gowns to 10 hospitals around the US
Thanks to our volunteers Mei Zhang, Xu Chen, Carrie Wang, Xiaoying Deng, Ted Lao, LingxiangZhou, Dennis Boos, Ami Patel, Barbara Young, Dan Castillo, QuanweiZhang, RuiminZhang, Yan Wang

See below table for list of cash donors that allowing CABS to disclose.
|
Last Name |
Donation ($) | Donation Time Stamp | Last Name | Donation ($) | Donation Time Stamp |
| Gan | 100 | March 22, 2020 4:23 PM | Wang | 60 | March 27, 2020 12:59 PM |
| Chen | 200 | March 22, 2020 4:29 PM | Zhu | 500 | March 27, 2020 6:35 PM |
| Du | 200 | March 22, 2020 4:43 PM | Jin | 40 | March 28, 2020 5:02 AM |
| Pei | 100 | March 22, 2020 4:47 PM | Yu | 200 | March 28, 2020 6:12 AM |
| Hao | 1,000 | March 22, 2020 4:51 PM | Yu | 400 | March 28, 2020 9:03 PM |
| Wang | 200 | March 22, 2020 5:40 PM | Cao | 200 | March 29, 2020 11:16 AM |
| Wang | 100 | March 22, 2020 6:35 PM | Wang | 200 | March 29, 2020 5:32 PM |
| Zeng | 40 | March 22, 2020 6:54 PM | Jiang | 2,000 | March 29, 2020 8:24 PM |
| Zhang | 60 | March 22, 2020 7:25 PM | Xu | 100 | March 30, 2020 2:39 PM |
| Tu | 200 | March 23, 2020 12:00 AM | Qian | 20 | March 30, 2020 8:21 PM |
| Yang | 100 | March 23, 2020 9:07 AM | Wu | 100 | March 30, 2020 9:53 PM |
| Wang | 400 | March 23, 2020 12:37 PM | Meng | 100 | March 30, 2020 10:19 PM |
| Ji | 200 | March 23, 2020 3:52 PM | Han | 20 | April 02, 2020 9:01 AM |
| Lin | 100 | March 23, 2020 5:01 PM | Miao | 500 | April 02, 2020 10:34 PM |
| Wei | 200 | March 23, 2020 5:44 PM | Wang | 100 | April 03, 2020 11:55 AM |
| Chen | 1,000 | March 23, 2020 7:06 PM | Zhang | 1000 | April 03, 2020 11:57 AM |
| Xu | 200 | March 23, 2020 7:46 PM | Virovek, Inc. | 5000 | April 03, 2020 12:05 AM |
| Yin | 20 | March 23, 2020 8:11 PM | Song | 20 | April 03, 2020 12:07 PM |
| Xu | 100 | March 23, 2020 8:29 PM | Zhong | 100 | April 03, 2020 12:09 PM |
| Zheng | 40 | March 24, 2020 10:14 AM | Wang | 60 | April 03, 2020 12:19 PM |
| Yang | 10 | March 24, 2020 4:58 PM | Zhou | 200 | April 03, 2020 12:46 PM |
| Li | 100 | March 24, 2020 6:29 PM | Cheng | 400 | April 03, 2020 2:03 PM |
| Ma | 100 | March 24, 2020 7:54 PM | Ma | 200 | April 03, 2020 3:27 PM |
| Wu | 400 | March 25, 2020 5:15 PM | Yu | 60 | April 03, 2020 5:45 PM |
| Tian | 200 | March 26, 2020 10:56 AM | Zhang | 100 | April 03, 2020 8:08 PM |
| Yan | 10 | March 26, 2020 4:34 PM | Wang | 200 | April 03, 2020 8:22 PM |
| Hu | 100 | March 26, 2020 4:44 PM | Zhang | 100 | April 03, 2020 9:38 PM |
| Lin | 500 | March 26, 2020 8:36 PM | Zhang | 100 | April 03, 2020 11:23 PM |
| Wu | 100 | March 26, 2020 10:22 PM | |||
| Wei | 100 | March 27, 2020 9:33 AM |

Many of you have heard, discussed or experienced a shortage of personal protective equipment (PPE) in US hospitals. This brings a big risk to our healthcare workers to combat COVID-19. CABS has decided to call for your donations to buy PPE and other essential supplies. Let's team up to fight against COVID-19!!!
1. 100% of your donation will be used to buy PPE or other essential supplies for local or other area US hospitals and clinics that have the most needs. CABS volunteers are working around the clock to secure PPE vendors and match hospitals or clinics. CABS will deliver goods to local (SF bay area) hospitals and clinics as priority but will also consider other areas if we have volunteers to facilitate the delivery.
2. Please Use a PC or laptop to make donations, click the "register" button at the up right or bottom of this web page. iPhone user may get an error message using our system but Android phone should be fine. Use a PC or laptop is the best way to donate through our website. Our online donation accepts credit cards. If you want to donate by personal check, please email yang.tian@cabsweb.org for instructions.
3. CABS is a 501(c)3 non-profit organization. You will receive a confirmation email of your donation, including CABS non-profit tax ID. You donation is fully tax-deductible. Please keep the confirmation email for your IRS tax filing purpose.
4. CABS will apply the most rigorous and diligent efforts to make sure we purchase qualified PPE and other supplies.
5. CABS will keep the entire donation campaign transparent. Total amount received, their usage, receipt from vendor and final accepting institutions, will be updated on our website as we go.
6. In case there is limited supply of qualified PPE and supplies that we can buy, any leftover donation will be cash transferred to local non-profit hospitals and clinics that need cash to fight COVID-19. Receipt from final accepting institutions will be updated at our website.
7. This donation campaign will end at April 4th (12 am), 2020
8. CABS will not provide any goods or service to you as a donor.
9. If you have any question, please email CABS President yang.tian@cabsweb.org
Sincerely
CABS Board of Directors, President and Executive Council
Online
ACROBiosystems is a leading manufacturer of recombinant proteins and other critical reagents to support the development of target therapeutics. The company employs an application oriented development strategy, with a particular focus on product design, quality control and solution based support. Our products and services enable anyone in the field of drug development to have a more intuitive and streamlined process.
Recently, ACROBiosystems has developed a series of 2019-nCoV/COVID-19 proteins to accelerate the development of drug, vaccine and diagnostic kits. This series include the high-quality ACE2, full length S, S1, S2 and other proteins with HPLC and SPR/BLI/ELISA validation. Check more details on www.acrobiosystems.com.
Thousand Oaks Biopharmaceuticals (TOBio) is an integrated CMC Solution Provider dedicated to large-scale GMP production of biologics with CDO and CMO capabilities. The TOBio Senior Leadership Team brings decades of experience and robust track records with expertise in cell culture media technologies, process development, and full CDMO services to build its high-quality platform for biologics development and manufacturing. As a trusted partner of the global biological industry, TOBio supplies innovative solutions and partners with biopharmaceutical facilities all over the world to meet tight timelines, drive down cost and provide top-level compliance.
Note:
- 1. This webinar is mainly oriented to specialists who are engaged in novel/generic products development and production in biopharmaceutical industry. It is also opens to community colleges and scientific institutions.
- 2. This webinar is a nonprofit event, free registration. However, only 400 seats are available.
- 3. This webinar playback is available. If you cannot attend the Webinar in real time due to time conflict, a registration for the playback link may be an option. Please follow us on LinkedIn for the update.
- https://www.linkedin.com/company/acrobiosystems-co-ltd
- 4. Registration liaison person:
Oscar Ouyang: oscar.ouyang@acrobiosystem.com
Celia Ma: celiatobiopharm@gmail.com
How to Register:
Click the link below to access the registration pathway:
webinar
CABS Webinar
Targeting Coronaviral Proteases with Small Molecule Drugs
US Pacific Time: March 16 (Monday), 2020. 3:30PM-5:00PM
China Beijing Time: March 17 (Tuesday), 2020, 6:30AM-8:00AM
Please click the link below to join the webinar:
From US: https://zoom.us/j/930185112
From China: https://zoom.com.cn/j/930185112
(China attendees, please download and install "Zoom Client for Meeting" from https://zoom.com.cn/download; then join meeting ID 930 185 112)
Or iPhone one-tap :
US: +16699009128,,930185112# or +16465588656,,930185112#
Or Telephone:
Dial(for higher quality, dial a number based on your current location):
US: +1 669 900 9128 or +1 646 558 8656
Webinar ID: 930 185 112
International numbers available: https://zoom.us/u/adlcq9ZV29 or https://zoom.com.cn/u/acpAsyQHOG
Agenda
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
3:30 pm - 3:40 pm Introduction to CABS, Yang Tian, President of CABS
3:40 pm - 4:40 pm Targeting Coronaviral Proteases with Small Molecule Drugs, Andrew Mesecar, PhD, Head of Biochemistry Department, Purdue University
4:40 pm - 5:00 pm Q&A
Speaker

Andrew Mesecar is the Walther Professor of Structural Biology and Head of the Department of Biochemistry at Purdue University. He also serves as the Deputy Director of the Purdue Center for Cancer Research. After the SARS outbreak in 2004, his lab started working on the discovery and structure-based design of inhibitors that target coronavirus 3C-like and papain-like proteases (PLpro) for the development of antiviral agents and attenuated vaccines. Working with Susan Baker at Loyola University, they were the first to discover that coronavirus papain-like proteases also function as deubiquitinating and deISGylating enzymes and that the structures of coronavirus papain-like protease domains are almost identical to the 58 known human ubiquitin specific proteases (USPs). In collaboration with his colleague Dr. Arun Ghosh (Purdue University), inventor of the FDA approved HIV protease drug Darunavir (Prezista®), his team has shown that coronaviral proteases (Plpro and 3CLpro) can be potently inhibited with small molecule compounds. They have developed a series of potent and selective PLpro inhibitors that are capable of inhibiting SARS-CoV in vitro and in a designed BSL2 animal model system. In addition, his team has developed a series of broad-spectrum inhibitors that inhibit the 3CLpro enzyme from over 11 different coronaviruses. They are currently testing their inhibitors and HIV and HCV protease inhibitors against PLpro and 3CLpro from SARS-CoV-2 that causes COVID-19. His studies support that these two proteases are viable drug targets and that their compounds may serve one day to treat coronavirus infections. Professor Mesecar has has authored over 135 peer-reviewed scientific publications and he has presented his research in over 150 seminars and poster presentations. His research is currently funded by grants from the National Institutes of Health and the Walther Cancer Foundation.
Webinar Abstract
Human coronaviruses such as SARS-CoV, MERS and SARS-CoV-2 continue to emerge as significant threats to public health. Other human coronaviruse such as NL63, HKU1, 229E and OC43 continue to persist in the population but are significantly less deadly. Due to the lack of any approved therapeutics for coronaviruses, the development of both vaccines and anti-viral drugs is paramount. HIV and HCV antiviral drug development teaches us that no single drug or class of drug target will suffice - we need multiple drugs in our arsenal to treat these diseases. The flu virus reminds us each year that we need to have both vaccines and small-molecule drugs available to control this deadly virus. Since the SARS-CoV epidemic emerged in 2003, we have worked to develop small-molecule inhibitors of coronavirus 3C-like protease (3CLpro) and the papain-like protease (PLP or PLpro). Initially, we focused on the proteases from SARS and then on NL63 and MERS. However, the differences in inhibitory potencies of our compounds and the taxonomic distance of the alpha and beta coronavirus genera taught us that approach of studying one virus at a time was too slow and provided to little molecular information to inhibit multiple coronaviruses. Moreover, it was not allowing us to predict how to inhibit emerging coronavirus pathogens. In the interest of pandemic preparedness, we are now taking what we call a taxonomically-driven approach to the structure-based design of coronavirus protease inhibitors. We targeted 3CLpro from the alpha-, beta- and gamma-coronavirus genera (FIPV, PEDV, NL63, HKU1, OC43, SARS, HKU4, HKU5, HKU9 and IBV) with a series of 50 compounds that we designed and synthesized using the Automated Synthesis and Purification platform at Eli Lilly. We identified inhibitor templates that potently inhibit the enzymes from the alpha and beta genera but not the gamma genus. To ascertain the structural basis of the selectivity, we used X-ray crystallography and a sparse-matrix sampling approach and determined multiple X-ray structures of 3CLpro in complex with different inhibitors from multiple CoVs. We have identified precise regions in the structures that define selectivity and we have extended this approach to PLpro.
Free registration here and is required to obtain Zoom Conference access link, which will be sent to your registration email before the webinar.
Recommend Using PC/Mac to Register. If CANNOT register using an iPhone, click here.
You can also register free at Eventbrite, click here to register.
Special Thanks to Zoom Video Communications and 北京幸福公益基金会 for technical support.


Zoom Conference
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
CABS Webinar Recap: Broad Spectrum Antiviral Remdesivir for the Treatment of Emerging Viral Infections with High Outbreak Potential
CABS successfully held its first webinar via Zoom conference on February 27th, 2020. Dr. Yang Tian, President of CABS, moderated this webinar. The webinar attracted more than 700 attendees from the U.S., China, and other countries. In this webinar, Dr. Danielle Porter, Director of Virology from Gilead Sciences, presented the preclinical development of Remdesivir; Dr. Tomas Cihair, VP of Virology from Gilead Sciences, discussed the clinical development of Remdesivir and ongoing clinical trials for COVID-19, which has caused more than 3,000 deaths globally since January 2020.

Dr. Porter presented the results relating to preclinical development and non-clinical antiviral effect of remdesivir in treatment of high breakout potential virus infections in animal models. Remdesivir is a broad spectrum antiviral drug that was discovered from Gilead library of nucleosides and nucletides as GS-441524, then further developed into a prodrug GS-5734 (Remdesivir) with improved antiviral activity against Ebola virus.
Non-clinical studies show Remdesivir’s in vivo efficacy for broad spectrum virus including filovirus, paramyxovirus and coronavirus. Testing preclinical treatment in animal models also demonstrated in vivo efficacy in SARS-CoV and MERS-CoV infected mice model. Furthermore, efficacy studies in Ebola virus-infected Rhesus monkey showed improved disease signs, indicating the potential therapeutic effects of Remdesivir for human infections. 
Dr. Cihlar discussed the clinical development of Remdesivir from 2015 to 2019, including the compassionate use of Remdesivir in DRC (MEURI Protocol), PALM trial -28-Day mortality according to treatment, and more recently, clinical trials of Remdesivir for the treatment of COVID-19. The ongoing COVID-19 clinical trial started in early February, 2020. Currently there are multiple phase 3 trials of Remdesivir for COVID-19 in China, US and other countries.

Finally, we sincerely thank Dr. Porter and Dr. Cihlar for giving a timely talk to CABS community, CABS EC volunteers for their efforts organizing this event, and Zoom Conference and Beijing Wellbeing Foundation for technical support and free Zoom access.
Zoom conference
CABS Seminar
Broad Spectrum Antiviral Remdesivir for the Treatment of Emerging Viral Infections with High Outbreak Potential
Agenda (February 27, 2020, Thursday)
(This event will be broadcast to you through Zoom Conference; agenda is based on US Pacific Time)
12:00 pm - 12:10 pm Introduction to CABS, Yang Tian, President of CABS
12:10 pm - 12:40 pm Discovery and Preclinical Data of Remdesivir, Danielle Porter, PhD, Director of Virology, Gilead Sciences
12:40 pm - 1: 10 pm Development of Remdesivir, Tomas Cihlar, Vice President of Virology, Gilead Sciences
1:10 pm - 1:30 pm Q&A
Webinar Zoom Access Link:
Join from a PC, Mac, iPAD or Mobile Phone via this link
Meeting URL: https://zoom.com.cn/j/540026848
Mobile one tap: US +16465588656,,540026848# or +16699009128,,540026848#
Join by Phone:
US +1 646 558 8656 or +1 669 900 9128
Meeting ID: 540 026 848
International Numbers: https://zoom.com.cn/u/auAXTwiBl
Due to large number of registrants, please join the webinar 5 minutes before the starting time (12pm US Pacific Time).
Any question? Please email info@cabsweb.org
Speakers

Danielle Porter, PhD, is a Director of Virology at Gilead Sciences. She obtained her undergraduate degree in biology at Dartmouth College and earned a PhD in virology from Harvard University. After spending several years at Novartis and then Lawrence Livermore National Laboratory, she joined Gilead in 2010. During her time at Gilead, she has worked on clinical trials for novel HIV and RSV therapeutics as well as emerging viral diseases. Most recently, she has been leading the nonclinical program for Remdesivir, which was recently studied in a clinical trial of acute Ebola disease in the Democratic Republic of the Congo and is now under investigation for efficacy against 2019-nCoV.

Tomas Cihlar, PhD, is a Vice President of Virology at Gilead Sciences with responsibility for coordinating preclinical antiviral research including projects on treatment, prevention, and cure of HIV, HBV, respiratory viruses, and emerging viruses. Dr. Cihlar joined Gilead after receiving his PhD in Biochemistry from the Institute of Organic Chemistry and Biochemistry (IOCB) in Prague, Czechia. Over the years, he has contributed to the development and regulatory approval of multiple Gilead’s antiviral products including all Gilead HIV drugs and their combinations. Together with his colleagues, he established research programs focused on long-acting antiretrovirals, in addition to a portfolio of projects aiming at the cure of HIV and HBV, and multiple programs for the treatment of respiratory and emerging/neglected viruses. Dr. Cihlar has served as a project lead for Gilead’s program on remdesivir, a broad-spectrum antiviral agent in clinical testing against Ebola infection and more recently against the 2019 new coronavirus. In past, Dr. Cihlar served on the Board of Directors of the International Society of Antiviral Research.
Seminar Abstract
Remdesivir (GS-5734) is a nucleotide prodrug with potent in vitro activity against multiple emerging virus families with high outbreak potential including filoviruses, coronaviruses and paramyxoviruses. The mechanism of remdesivir’s antiviral activity is through delayed chain termination of viral RNA transcription following the incorporation of the active intracellular nucleoside triphosphate metabolite into RNA as shown in biochemical assays using recombinant Ebola, Nipah, and respiratory syncytial virus (RSV) RNA dependent RNA polymerases. Remdesivir has demonstrated in vivo efficacy against Ebola virus in non-human primates which has led to its inclusion in clinical studies evaluating the effects of remdesivir treatment in acute Ebola virus disease (EVD) as well as in male EVD survivors with prolonged viral shedding in semen. The efficacy of remdesivir against other emerging viral infections has been demonstrated in animal models for Marburg virus, Nipah virus, sudden acute respiratory syndrome (SARS) coronavirus, and Middle East respiratory syndrome (MERS) coronavirus. Together, the current preclinical and clinical profiles of remdesivir support its further development as a broad spectrum antiviral to manage emerging viral infections with high mortality and significant outbreak potential including the novel coronavirus, 2019-nCoV.
Special Thanks to Zoom Video Communications and 北京幸福公益基金会 for technical support.


Online Webinar through Zoom Conference
BioPacific Toastmasters club contests of 2020
Please join us at the BioPacific Toastmasters club contests of 2020! It will be a guaranteed event of excitement and joy as you will see our senior club members applying their best efforts in the contests. You can participate by signing up for functional roles including ballot counters and timers or simply as a guest to learn more about our club and the Toastmasters community. You can find the agenda and sign-up link for each contest in the Meetup links below.
We will be providing FREE breakfast, snacks, and drinks!
BioPacific Toastmasters Speech Contest
What is it: Contestants will present their prepared speeches to the audience
Time: 9:45 am - 11:15 am, Feb 22nd, 2020
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Meetup link: https://www.meetup.com/district4toastmasters/events/qspcqqybcdbdc/
BioPacific Toastmasters Open house and Evaluation Contest
What is it: Contestants will present their evaluation for the same speech delivered by our test speaker
Time: 9:45 am - 11:15 am, Feb 29th, 2020
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Meetup link: https://www.meetup.com/district4toastmasters/events/qspcqqybcdbmc/


Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
.tiff)
.png)

Kay Tong: Kay currently is the Senior Director of Quality Assurance at Apollomics. Inc. Before joining Apollomics, Kay served as Global Process Leader for Laboratory Investigations at Gilead Science and as Head of Quality for a business unit at Life Technologies. Kay also has been a business owner as a Quality Consultant for over 8 years. While at Gilead, Kay served in the Gilead Circles Mentor-ship program and several mentor-ship programs. Kay has guided a number of personnel for successful promotions, career changes as well as salary negotiation.

Jonathan Wong: Jonathan currently oversees the Talent Acquisition organization for Exelixis (www.Exelixis.com, EXEL, S&P MidCap 400 index), which is an Oncology focused Biotech company that develops and commercializes therapies for the treatment of particular type of cancers, more specifically RCC (a type of kidney cancer) and HCC (a type of liver cancer) in addition to other potential indications within Oncology. Jonathan’s group leads the overall recruitment for Exelixis across all phases (Drug Discovery, Clinical Trial Development, Commercial Marketing, Legal and IT functions etc.). In addition, Jonathan’s group owns talent analytics/reporting, digital media, vendor management and company headcount forecast/planning.
Due to potential risk of Coronavirus infection, this event is postponed to a later date (to be determined...). We will update later.
1633 Old Bayshore Hwy Suite 280 Burlingame, CA 94010
If you are interested in volunteering this activity, please register by click the 'Register" button at the bottom. We will follow up with you for more details.
疫情爆发,举国同心。武汉地区爆发新型冠状病毒肺炎,医院拥挤、医生劳顿、物资急需、病情不明,疫情对医护人员、病患人群、广大民众产生心理影响。北京幸福公益基金会在前期参与急需物资筹集和运送的过程中,于大年初一凌晨收到“武汉一线医护人员急需心理支持“的紧急求援!北京幸福公益基金会在清华大学心理学系、清华大学社会治理与发展研究院、清华大学幸福科技实验室Hlab、Zoom视频通讯有限公司的大力支持下,于1月25日启动“心系武汉”紧急心理援助项目。本项目将动员和组织理学专家和心理专业志愿者,以专业性、有效性、持久性为原则,支援武汉!
“心系武汉”心理援助开展以下三项行动:
1、成立心理专家与专业志愿者团队 由具有丰富经验的专家组成专家团队,提供专业培训和督导。组织具有心理学资质的志愿者,经专项培训后为武汉当地医护人员、病患和大众开展社会心理科普、一对一心理辅导等,并提供专业督导。
2、提供技术支持平台 为心理专家和心理援助者提供在线技术支持,鉴于疫情严重,本次心理援助工作全部在线上以视频或语音形式进行。目前Zoom视频通讯有限公司提供了全面技术支持,提供足够免费帐号和线上视频系统,供心理援助志愿者使用。
3、提供资金支持 支持技术平台的建设和维护所需资金,支持专业培训与心理援助等相关费用。
“心系武汉“心理援助项目招募志愿者如下:
1、心理援助志愿者:通过线上为一线心理专业志愿者提供专项培训,并进行督导、监测和评估等专业支持;通过线上对疫区人员开展心理科普、心理辅导、心理咨询等;编撰疫区心理援助专业资料。
2、 线上技术支持:协助线上技术实施,协助专家和志愿者使用线上工具,协助操作,解决技术难题。
3、运营组织协调:协助安排专业培训,协助求助者与心理援助者联络与沟通,协助其他事务性工作。
志愿者条件和承诺:
1、具有心理学、医学、教育学、社会学、社会工作等相关专业和工作经验。
2、自愿参与心理援助工作,遵循心理学工作原则,秉持志愿精神,热心为他人服务。
3、志愿工作时长一周内不少于2小时。
online
About current Coronavirus outbreak, what CABS can do?
Dear CABS community:
I believe everyone is concerned about the novel Coronavirus (2019-nCov) outbreak in Wuhan, China. CABS is no difference. CABS Executive Council team has been closely monitoring the situation and actively discussing what we can help. Here are a few things we want to share with you.
1. Dose CABS accept donations to buy critical medical supplies for China?
Currently CABS doesn't accept donations for the purpose of buying critical medical supplies for China. This is because most of the critical items, e.g., medical face masks and protection clothes, are out of stock for most US distributors and manufacturers. Major manufacturers will ramp up production in China and distribute directly in China. There is very limited supply in the US that we can buy. We have confirmed that many sister organizations are facing similar challenges. CABS will closely monitor the situation and update our community when we are ready to accept donations for relieving 2019-nCov outbreak.
2. What is the plan for upcoming CABS events?
Public health and safety is always the first priority for CABS events. We have cancelled CABS Chinese New Year Party that was planned on Jan. 26th. We also postponed all upcoming CABS events in February and March. CABS will closely monitor the situation and update our next event schedule through our website and weekly newsletter.
3. What is CABS recommendation on prevention of 2019-nCov infection?
CABS recommends everyone to get the latest updates and guidance from US CDC website (https://www.cdc.gov/coronavirus/2019-ncov/index.html).
A. CDC guidance in English: https://www.cdc.gov/coronavirus/2019-ncov/guidance-prevent-spread.html
B. CDC guidance in Chinese: https://www.cdc.gov/coronavirus/2019-ncov/guidance-prevent-spread-chinese.html
C. CDC guidance on traveling to China: https://wwwnc.cdc.gov/travel/notices/warning/novel-coronavirus-wuhan-china
4. CABS is recruiting volunteers to write scientific/educational articles on discovery and development of medicines for the prevention and treatment of Coronavirus infection.
Most of CABS members are professionals in drug discovery industry. This is where we can help. We are looking for volunteers to write short scientific articles (1-3 pages with figures/tables and text) on the following topics. The articles will be shared with CABS community by weekly newsletter and WeChat. We hope this effort could advocate more discovery efforts on potential therapeutics for Coronavirus.
A. Discovery of Coronavirus entry inhibitors (antibody, peptides, purified proteins or small molecules)
B. Discovery of Coronavirus protease inhibitors
C. Discovery of Coronavirus RNA polymerase inhibitors
D. Discovery of Coronavirus assembly and secretion inhibitors
E. siRNA for Coronavirus
F. Immunotherapy for Coronavirus (vaccine and others)
G. Diagnostics for Coronavirus
H. In vitro screening for inhibitors of Coronavirus
If you are interested in writing scientific articles on above topics or you have additional topics/ideas, please email yang.tian@cabsweb.org for details.
CABS is together with all of you, to contribute our efforts curbing Coronavirus outbreak!
Please feel free to email us any other question and suggestion: info@cabsweb.org
Sincerely
Yang Tian
CABS President
关于当前的新冠状病毒爆发,CABS能做什么?
尊敬的CABS社区:
我相信每个人都对在中国武汉爆发的新型冠状病毒(2019-nCov)感到担忧。 CABS也和你们一样。 CABS执行理事会团队一直密切关注局势的发展,并积极探讨我们可以提供的帮助。在此,我们想与您分享一些目前的想法及计划。
一. CABS是否接受捐款用于为中国购买必要的医疗用品?
目前,CABS不接受用于为中国购买必要医疗用品的捐款。原因是来自大多数美国分销商和制造商的必要医疗用品(例如医用口罩和防护服)目前都缺货。而且,目前的趋势是这些医疗用品的主要制造商将提高在中国的产量,并直接在中国分销。在美国,我们可以购买的货源非常有限。我们已经和许多姊妹组织确认,他们都面临着类似的挑战。CABS将密切关注局势的发展,并根据情况更新和调整是否需要大家的捐款来缓解2019-nCov疫情。
二. CABS对将举行的活动有什么计划?
公共卫生与安全始终是CABS活动中优选考虑的第一要素。为此,我们已取消原定于1月26日举行的CABS农历新年晚会。我们还推迟了CABS在2月和3月份的活动。 CABS将密切关注局势的发展,并通过我们的网站和每周新闻通讯更新我们的下一个活动时间表。
三. CABS对预防2019-nCov感染有什么建议?
CABS建议大家从美国疾病预防控制中心网站获取最新更新和指南(https://www.cdc.gov/coronavirus/2019-ncov/index.html)。
A. CDC英文指南:https://www.cdc.gov/coronavirus/2019-ncov/guidance-prevent-spread.html
B. CDC中文指南:https://www.cdc.gov/coronavirus/2019-ncov/guidance-prevent-spread-chinese.html
C. CDC对前往中国旅游的指南:https://wwwnc.cdc.gov/travel/notices/warning/novel-coronavirus-wuhan-china
四.CABS正在招募志愿者撰写与开发用于预防和治疗冠状病毒感染的药物相关的科普和教育类文章。
CABS的大多数成员是药物研发的专业人员,这是我们的优势也是我们最可以提供帮助的地方。我们正在寻找志愿者撰写以下主题的简短科普文章(包括图表和文字共1-3页)。这些文章将通过网站、每周新闻通讯和微信公众号与CABS社区共享。我们希望这项工作有助于对冠状病毒的潜在疗法有更多的发现。
A. 冠状病毒侵染宿主细胞的抑制剂(抗体、多肽、纯化蛋白质或小分子)
B. 冠状病毒蛋白酶抑制剂
C. 冠状病毒RNA聚合酶抑制剂
D. 冠状病毒装配和分泌抑制剂
E. 针对冠状病毒的siRNA
F. 冠状病毒的免疫疗法(疫苗和其它)
G. 冠状病毒的诊断
H. 冠状病毒药物体外筛选模型的建立和使用
如果您有兴趣撰写有关上述主题的科普文章,或者您有其它主题/想法,请发送电子邮件至yang.tian@cabsweb.org。
CABS和大家一起,为遏制冠状病毒的爆发做出我们最大的努力! 如果您有其它任何问题和建议,请发送邮件至:info@cabsweb.org。
谢谢!
田洋博士
CABS会长
Due to potential risk of Coronavirus infection,
CABS CHINESE NEW YEAR GALA IS CANCELLED!!!
If you already registered, we will refund you!
2020 will be the year of Metal Rat. Ranking the first in the Chinese zodiac, rat represents wisdom. To celebrate the Chinese New Year, CABS (Chinese American Biopharmaceutical Society) is planning a Gala lunch and show event to warmly welcome new friends and to sincerely thank generous supporters throughout the years. Please join us on Sunday, January 26th to kick off the Year of the Rat with delicious Chinese lunch followed by exciting shows!

Program includes:
Folk and pop songs, guitar performance, fashion show, martial art, allegro (快板), poetry recitation, talk-show, flute performance, dancing, magic show, and so much more! Meanwhile, there will be many prizes for solving Chinese conundrums and several rounds of lucky draws of Amazon gift cards! Come to meet old friends, make new friends and have tons of fun!
Registration Requirement:
Online: $15: Adult (13 and older); $10: Senior (65 and older); $5: Kid (12 and under)
Onsite: $20: Adult (13 and older); $15: Senior (65 and older); $10: Kid (12 and under)
Please mark your calendar, and your early online registration will be greatly appreciated. We look forward to seeing you there and celebrating Year of the Rat together!
CABS sincerely appreciates sponsors of this event for their generous support!


Recreation Center, 650 Shell Boulevard, Foster City, CA 94404
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Chinese American BioPharmaceutical Society (CABS) is pleased to announce that 2020 CABS Investor Forum will be held on January 15th of 2020 in conjunction with JP Morgan Healthcare Conference. This forum will feature a morning session and an afternoon session. The morning session will be joined by China- and US-based investors, business development executives, and entrepreneurs. The topics will focus on the trends, opportunities and challenges in capital investment, business development and M&A, in US and China Biopharma.
The Entrepreneurship Club (E-Club) of the Chinese American Biopharmaceutical Society (CABS) is proud to present its annual Innovation Roadshow at 2-4:30 pm on January 15, 2020 in Morrison & Foerster’s San Francisco office. The Innovation Roadshow serves the dual purposes of celebrating the entrepreneurship tradition of the CABS community and providing a debut stage for emerging entrepreneurs of the community. This year, eight start-up companies have been selected to showcase their innovative technologies. This event is held in conjunction with the CABS Investor’s Forum, a highly popular workshop that explores topics related to US/China cross-border issues in the biopharmaceutical industry, during the JP Morgan week.
1. Photo ID is required to enter the building
2. If you registered for "Morning Session Only" or "Both Morning and Afternoon Sessions", please arrive and check-in before 10AM. After 10AM, the remaining seats will be assigned to waitling list poeple who are waiting at the building entrance
3. If you registered for "Afternoon Session Only", please check-in between 1-3PM. You will NOT be admitted to morning session. After 3PM, the remaining seats will be assigned to waitling list poeple who are waiting at the building entrance
4. No Walk-in Allowed; Only online registrants will be allowed admission!!! Waiting list will be allowed admission 10am-10:30am or 3-3:30PM and depend on seats availability.
Agenda
Morning Session
8-8:50 am: Registration and Networking
8:50-9:00 am Opening Remark by Yang Tian, CABS President
9:00-9:20 am Jonathan Norris, Managing Director, Silicon Valley Bank, Overview of US Biopharmaceutical Investment, Corporate Business Development and M&A.
9:20-10:20 am Panel discussion 1: Trends, Challenges and Opportunities in US Biopharma Investment
Moderator: Janet Xiao, Partner, Morrison & Foerster LLP
Confirmed Panelists:
Jonathan Norris, Managing Director, Silicon Valley Bank
Ori Solomon, Partner, Morrison & Foerster LLP
Hong Zheng, VP, Ally Bridge
Tina Yu, CEO, Mevion
Faz Bashi, Venture Affiliate Partner at Boston Millennia Partners
10:20-10:50 am: Coffee Break and Networking
10:50-11:10 am Greg Scott, Founder, ChinaBio, Overview of China Biopharmaceutical Investment, Corporate Business Development and M&A
11:10-12:00 pm Panel Discussion 2: Trends, Challenges and Opportunities in China Biopharma investment (tentative panel theme)
Moderator: Alex Zhang, CEO, Hanhai Silicon Valley
Confirmed Panelists:
Dandan Dong, Managing Director, Vivo Capital
Karen Liu, Founding Partner, 3E Bioventures Capital
Greg Scott, Founder, ChinaBio
Lusong Luo, Sr. Vice President, BeiGene
12:00-1:00 pm Lunch and Networking1:00 pm End of morning program
Afternoon Session
1:30-2:00 pm Registration
2:00-4:30 pm Innovation Roadshow
Moderator: Huijun Zhou, CABS Entrepreneurship Club Program Chair

Morning Session Panelists

Morning Session Panelists
Jonathan Norris, Managing Director, Silicon Valley BankDr. Jonathan Norris is a managing director for the healthcare and life science practice for Silicon Valley Bank. Norris oversees business development efforts for banking and lending opportunities as well as spearheading strategic relationships with many life science and healthcare venture capital firms. He also helps SVB Capital through sourcing and advising on limited partnership allocations. In addition, he speaks at major investor and industry conferences and authors widely cited analyses of healthcare venture capital trends. Norris has more than sixteen years of banking experience working with healthcare companies and venture capital firms. Norris earned a bachelor's degree in business administration from the University of California, Riverside and a juris doctorate from Santa Clara University.
Ori Solomon, Partner, Morrison & Foerster
Ori Solomon is a partner in the Corporate Department and Co-chair of the firm’s Emerging Companies and Venture Capital practice.Mr. Solomon provides general corporate representation to a wide range of companies, from start-ups and emerging growth companies to large public companies. Mr. Solomon also represents venture capital funds in a wide range of transactions, including investments in portfolio companies and secondary direct transactions.His transaction-based practice focuses primarily on representing emerging companies and their investors in a variety of venture and growth equity financings, secondary investments, mergers and acquisitions, joint ventures and other strategic transactions. Mr. Solomon has represented clients in a variety of industries, primarily life sciences and technology. He is recognized as a leading lawyer by Legal 500 US for Media, Technology & Telecoms.Mr. Solomon also regularly represents both buyers and sellers in structuring, negotiating and documenting acquisitions involving public and private companies, and also has significant experience in public and private corporate finance transactions, including registered equity offerings, PIPEs and other alternative financing transactions. He regularly advises public company clients with respect to corporate governance issues, board of directors matters, SEC periodic reporting and other securities law compliance, proxy statements and annual meetings, disclosure controls and procedures, and Sarbanes-Oxley and other related matters.
Hong Zheng, PhD, Vice President, Ally Bridge Group
Dr. Hong Zheng is currently a Vice President at Ally Bridge Group (New York). He leads the investment research in the US Biotechnology public market. Dr. Zheng has over 10 years of experience in biomedicine, working across the fields of investment, industry, and academia. Before joining Ally Bridge Group, Dr. Zheng was a senior equity research associate at RBC (Royal Bank of Canada) Capital Markets, when he covered over 30 US biotech companies. Prior to RBC, Dr. Zheng was a lead chemist at Ra Pharmaceuticals (recently acquired by UCB), where he led the med-chem efforts of multiple drug discovery programs including small molecule and peptide drug candidates. Dr. Zheng completed his Bachelor’s degree at Fudan University and received his Ph.D. in Chemical Biology from Boston College. He is the lead author of numerous highly impactful research articles in top-ranked journals and the key inventor of several patents.
Janet Xiao, PhD, JD, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Janet focuses her practice on worldwide patent procurement, patent portfolio management, and strategic planning for life sciences companies. She advises her clients on patent matters relating to various technologies, including antibody therapeutics, cell therapeutics, nanomedicine, gene therapy, drug delivery systems, diagnostics, and nutraceuticals. Recognized by Chambers as being highly sought after for patent prosecution and strategy mandates by innovators from around the world, Janet develops and strengthens her clients’ complex patent portfolios to maximize their commercial value. She is instrumental in developing strategies for multibillion-dollar patent portfolios for pharmaceutical clients. Janet works extensively in performing IP due diligence reviews in the contexts of VC investments, technology transactions, mergers and acquisitions, and marketing and manufacturing clearance for biopharmaceutical products. She helps some of the world’s most-sophisticated biopharmaceutical companies and investors assess potential risks and devise preemptive risk-management strategies for their product development and investments. She has led hundreds of IP due diligence projects, and is noted for her in-depth knowledge of patent landscapes in the fields of cancer immunotherapy, gene editing, next-generation sequencing, liquid biopsy, antibody manufacturing, and medical devices. Janet works regularly with patent litigators in the event of legal challenges by third-party competitors or vice versa, and she is frequently called upon to represent clients in litigation and post-grant proceedings. She is among the very few IP attorneys in the world who are both equipped with solid skills in global strategic IP management and knowledgeable about IP issues unique to China and Chinese clients, and she has been the go-to counsel for IP issues in U.S./China cross-border transactions and operations in China. Since 2005, Janet has been actively involved in the leadership team of the Chinese-American Biopharmaceutical Society (CABS), a nonprofit organization dedicated to bridging the life sciences communities in the U.S. and China, including serving as president for the 2011–2012 term.
Greg B. Scott, Founder and Chairman, ChinaBio® Group
Greg founded ChinaBio® Group in 2007 to help life science companies and investors achieve success in China. ChinaBio® works with US, European and APAC companies seeking partnerships, acquisitions, novel technologies and funding in China. ChinaBio® has also organized over 30 conferences in China focused on cross-border investment and partnering, including the ChinaBio® Partnering Forum which draws over 1400 attendees from around the world to China each spring. Greg is also co-founder of two investment groups that have funded over 50 biotechnology and medical device companies in the US and China, and Executive Editor of ChinaBio® Today, a widely read newsletter covering the China life science industry. He also is the current chair for the American Chamber of Commerce Healthcare Committee, and a former board member of BayHelix Group. Greg is considered a leading expert on China’s life science industry and is frequently quoted in media including the Wall Street Journal, Financial Times, Bloomberg, BioWorld, BioCentury, and other industry publications. Headquartered in Shanghai, ChinaBio® has team members in San Diego, Palo Alto, and Basel, Switzerland.
Tina Yu, PhD, Co-Founding Partner of YuanMing Capital
Dr. Tina Tianning Yu has almost twenty years of professional experience working in the life sciences sector and the financing world of the healthcare industry. Dr. Yu is a Co-Founding Partner of YuanMing Capital, a healthcare specialty fund and CEO of Mevion Medical Systems. She led the investment in BeiGene, Ascentage Pharmaceuticals, and Mevion Medical Systems, etc. Prior to that, Dr. Yu worked for Vision Capital, a NYC-based investment management firm, as a Director of Investment, responsible for US biotech and China sectors. Before becoming an investor, Dr. Yu was a senior research scientist at Wyeth BioPharma, where she specialized in therapeutic protein development. Dr. Yu serves on the Board of Mevion Medical Systems, Alliance for Proton Therapy Access and USTC Alumni Association in Greater New York. She is a regional director of HBS Healthcare Alumni Association. Dr. Yu holds a PhD in Chemistry from Princeton University, an MBA from Harvard Business School, and a B.S. in Biology from University of Science and Technology of China.
Dandan Dong, PhD, Managing Director, Vivo Capital
Dandan Dong, Ph.D., is a Managing Director of Vivo Capital. She has a background in academic biomedical science research and translational medicine. She has more than eight years’ experience in healthcare investments in both the United States and China. As a member of Vivo’s cross-border strategy, she has been involved in multiple transactions in both countries and cross-border deals. She is the founding member of VISEN Pharmaceuticals and currently serves as VISEN’s Chief Business Officer. She is a managing member of Vivo PANDA Fund, Vivo’s early-stage investment vehicle. Dr. Dong holds a Ph.D. in biomedical science from Fudan University and she has completed the Pre-doctoral Fellowship program at New York University School of Medicine.
Karen Liu, PhD, Founding Partner of 3E Bioventures Capital
Dr. Karen Liu has been active in China healthcare venture investment since 2005. She is a founding partner of 3E Bioventures Capital, a healthcare-focused venture investment firm actively investing in worldwide new drugs and innovative medical devices and diagnostics.Prior to 3E Bioventures, Dr. Karen Liu was a partner of Kaiwu Capital and Managing Director at CRCI Capital, in charge of healthcare investment. Dr. Karen Liu received her Ph.D in Immunology and Master of Medical Sciences degree from Harvard University, and Bachelor of Sciences degree from Cornell University. She also received an EMBA degree from CKGSB-Chungkong Graduate School of Business in China.
Lusong Luo, PhD, Sr. Vice President, BeiGeneDr. Lusong Luo is currently SVP & Head of External Innovation at BeiGene. Prior to taking this role, Dr. Luo is SVP & Head of Discovery Biology at BeiGene. Dr. Luo is one of the founding scientists of BeiGene. Before joining BeiGene, Dr. Luo held positions in BioDuro as Sr. Director and Director of Discovery Biology. Dr. Luo has also spent a number of years at GlaxoSmithkline Oncology CEDD, where he served as Group Manager, Research Investigator, and Principal Scientist. Dr. Luo was a key member of a number of oncology drug discovery/development teams that have contributed to the discovery and development of around a dozen clinical compounds over the years. Dr. Luo has published over 50 research papers, reviews, and book chapters. He was trained with Professor. Christopher T Walsh at Harvard Medical School and obtained his Ph.D. in Biochemistry from University of New Mexico.
Alex Zhang, PhD, MBA, CEO of Hanhai Silicon Valley
Alex Zhang is the CEO of Hanhai Silicon Valley., an early stage investor and cross-border accelerator for startups in San Francisco Bay Area. Prior to Hanhai, Alex was the Managing Partner of Enverest, LLC., a Silicon Valley-based innovation solutions and investment advisory firm with branch offices in China and Singapore. Prior to co-founding Enverest, Dr. Zhang spent nearly five years at Thermo Fisher Scientific, where he was responsible for 4 business development deals exceeding $10 M, and played a key role in several billion-dollar acquisitions in MedTech. From 2001 to 2009, Dr. Zhang was a Senior Scientist at Tularik Inc. (acquired by Amgen in 2004), where he led drug discovery endeavors in oncology, cardiovascular and metabolic diseases therapeutic areas. Dr. Zhang received MBA degree at Cornell University, PhD in Organic and Analytical Chemistry at Texas A&M University, and BS in Chemistry at Shandong University. During his graduate research, he focused on the design and synthesis of therapeutic peptoids, as well as biological mass spectrometry. His research has led to publication of more than 20 peer reviewed articles and 4 patents. Dr. Zhang has served multiple leadership roles in the Executive Council of CABS, including as the President in 2017-18. Over the past decade, Dr. Zhang has advised a number of successful MedTech and digital health startups based in Silicon Valley.
Faz Bashi, MD, Venture Affiliate Partner at Boston Millennia Partners
Faz K. Bashi, MD, has a research background in Immunology and Virology from UCSF. He is an active member of Life Science Angel's (LSA) in Silicon Valley. He is a member of Berkeley Angel Network. He is Lead Venture Investor with Portfolia's FemTech Fund focused on female health and wellness. He is West Coast Affiliate Venture Partner at Boston Millennia Partners focused on digital Health. He served a 3-year term as Board Member of the Angel Capital Association (ACA) where he continues to be Chair of the ACA's Life Sciences Syndication group. He is active with UCSF’s Entrepreneurship Startup 101 and Lean Launchpad, external advisory board member for UCSF's CTSI and Johns Hopkins' CBID (https://cbid.bme.jhu.edu/), Mentor for women entrepreneur leaders at both CSweetener.org and Springboard Enterprises (www.sb.co) Life Sciences Council. He volunteers his time with Peninsula Bridge program to help the underserved kids of East Palo Alto get inspired about their futures via education. He is committed to helping find treatments for Tinnitus, Autism, Lupus, and Vascular diseases. He completed several triathlons with Team-In-Training to raise funds for the Leukemia and Lymphoma Society. Currently he is very active in Pet Health, and serves on the Board of Directors at Anubis Bio (www.anubisbio.com), developing an all-natural medical food that saves puppies from dying due to parvovirus and bacterial causes of gastrointestinal distress leading to diarrhea.
1. Photo ID is required to enter the building
2. If you registered for "Morning Session Only" or "Both Morning and Afternoon Sessions", please arrive and check-in before 10AM. After 10AM, the remaining seats will be assigned to waitling list poeple who are waiting at the building entrance
3. If you registered for "Afternoon Session Only", please check-in between 1-3PM. You will NOT be admitted to morning session. After 3PM, the remaining seats will be assigned to waitling list poeple who are waiting at the building entrance
4. No Walk-in Allowed; Only online registrants will be allowed admission!!! Waiting list will be allowed admission 10am-10:30am or 3-3:30PM and depend on seats availability.
Cannot Register Using iPhone? Click Here
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105-2482

China’s healthcare sector has become a vast ecosystem, taking up the world’s second-largest pharmaceutical and medical device market. With China’s healthcare industry projected to be worth $2.4 trillion by 2030, its maturity from infancy will be tied to growing domestic demand and expanding its global reach.
However, there are still a few challenges for companies to do cross-border business with Chinese partners. First, most companies and entrepreneurs are worried about one big issue in China: intellectual property protection. The good thing is China's intellectual property protection has already been comprehensively strengthened every year. Trade War is another big concern about cross-border business since 2018.
Case studies for cross-border partnership, business, and investment opportunities would be the focus of this event.
In addition, there are over 400 medical and biotechnology companies and investors from around the world coming to the summit to find cooperation and investment opportunities through live roadshows and 1-on-1 private talks.
The detailed program can be found at: https://jpm2020.button.tech/
Julia Morgan Ballroom @ The Merchants Exchange 465 California Street, 15th Floor San Francisco
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Please join us to celebrate the 2019 Career Advisory Network (CAN) graduation event (For details please see the flyer). This year, we invited 2 outstanding speakers who will share their experience and advice on career development and success.

For catering purposes and limited space, please kindly register by 12/05 for this event using the link: https://docs.google.com/forms/d/e/1FAIpQLSeD_53ud_HBM-p81GyUH82i1lhyFvMlxRevQ4Dfl7cWSKwtAg/viewform?usp=pp_url
Hanhai Biolabs (1633 Old Bayshore Hwy Suite 280, Burlingame, CA 94010)
CABS Entrepreneurship Club Annual Thanksgiving Party
Agenda:
12:00-12:10 Dr Tian Yang, President of CABS Opening Remarks;
12:10-12:25 Dr Zhou Huijun, Program Chair of CABS Entrepreneurship Club
Overview of CABS Entrepreneurship Club and upcoming events
12:25-12:30 Dr. Alex Zhang, CEO of Hanhai Lab
12:30-14:00 Lunch and 30-Second Elevator Speech for Self Introduction
14:00-15:00 Networking
Space is limited. Please register soon.
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
You are cordially invited to Chinese American BioPharmaceutical Society (CABS) Entrepreneurship Club Annual Thanksgiving Party
Agenda:
12:00-12:10 Dr Tian Yang, President of CABS Opening Remarks;
12:10-12:25 Dr Zhou Huijun, Program Chair of CABS Entrepreneurship Club Overview of CABS Entrepreneurship Club and upcoming events
12:25-12:30 Dr. Alex Zhang, CEO of Hanhai Lab
12:30-14:00 Lunch and 30-Second Elevator Speech for Self Introduction
14:00-15:00 Networking
Space is limited. Please register soon.
Cannot Register Using iPhone? Click here

1633 Old Bayshore Hwy Suite 280, Burlingame, CA 94010
CABS Entrepreneurship Club Celebrates Thanksgiving Holiday
Old and new friends of CABS’ Entrepreneurship Club (“E-Club”) gathered on Sunday November 24, 2019 at Hanhai Lab’s Burlingame campus to celebrate the beginning of this year’s holiday season. As a popular annual event, the E-Club’s Thanksgiving Party attracts a wide range of interested individuals with diverse backgrounds and expertise, including seasoned entrepreneurs, investors, founders of startup companies, R&D scientists, consultants, and lawyers. This year’s event received generous sponsorship from Protheragen, Inc., an innovative drug research and development project incubator based in China and the United States.
Dr. Tian Yang, President of CABS, started the event with opening remarks. Dr. Huijun Zhou, E-Club’s Program Chair, followed with an overview of the E-Club, highlighting signature E-Club events that offer opportunities for talents from the CABS community to connect and collaborate. Dr. Alex Zhang, CEO of Hanhai Lab, also provided an introduction of Hanhai Lab’s mission and resources across the globe.


Event attendees mingled while enjoying delicious Chinese food and a roasted turkey. Each attendee delivered a 30-second pitch. Some talked about their professional aspirations and ventures, while others shared their personal interests and hobbies. New connections were made, opportunities were announced, and collaborations were called for—all with festivity in the air.


E-Club looks forward to keeping in touch with all members of the CABS community who share the inner calling of entrepreneurship. E-Club wishes you a happy holiday season and a prosperous new year.
About CABS Entrepreneurship Club
The Chinese American Biopharmaceutical Society (CABS) is a non-profit organization for professionals in the biopharmaceutical industry. CABS is headquartered in San Francisco Bay Area, California. CABS is a highly influential association with more than 3000 members and subscribers in the life sciences industry. CABS is the largest and most active Chinese biopharmaceutical association in Northern America. CABS Entrepreneurship Club helps entrepreneurs in the CABS community and foster entrepreneurship, innovative culture, and business opportunities in the life science industry between the U.S. and China.
Special thanks for this event sponsor Protheragen Inc.


Protheragen is an innovative drug research and development project incubator based in China and the United States. It focuses on investing in early-stage new drug development projects, especially those which are not marketed or not patented or even without data. Protheragen cherishes each and every brilliant idea brewed in the minds of scientists. Protheragen is probably the only incubator in the world dedicated to helping scientists, who by themselves are unable to start a company and develop innovative drug development projects. Protheragen aims to invest in 200 early-stage new drug research and development projects at $ 1-3 million or more per project.
Organization:TOPS Annual Symposium: Translating Discoveries to Cures, Stanford University
Details&Registration:http://med.stanford.edu/cancer/research/research-programs/translational-oncology/TOPS-Symposium.html
Stanford University, Berg Hall, Li Ka Shing Center (LKSC)
Organization: CBA
Theme: From Disruptive Innovations to Real-World Applications
Topics: Innovative Drug Discoveries and Diagnostic Technologies; AI and Big Data in Healthcare; Global Regulatory Considerations, Healthcare Investment and M&A etc.
Details&Registration: https://www.cbasf.org/october-12-2019-the-22nd-cba-annual-conference/

Crowne Plaza Hotel, 1221 Chess Drive, Foster City, CA 94404
Gene Therapy Workshop Event Recap

Gene Therapy Workshop, co-organized by CABS (the Chinese American Biopharmaceutical Society) Science & Technology Committee and CCBEA (California Chinese Biotech/Biopharmaceutical Entrepreneur Association), was successfully held on October 5, 2019 at Hanhai BioLabs in Burlingame, CA. Nearly 150 professionals attended the workshop that included 5 talks given by gene therapy experts from both industry and academia. The topics covered lentivirus gene therapy, Adeno-Associated Virus (AAV) gene therapy manufacturing, and non-viral gene deliver using nanoparticles.
Dr. Yan Wang, the Past President of CABS gave welcome remarks and introduced CABS briefly. Dr. Shenjiang Liu, the President of CCBEA, introduced the history of CCBEA and reviewed the main activities of CCBEA in the past years. Dr. Xiang Yi and Dr. Ken Zhang co-chaired this workshop.


Dr. Gary Li, the founder and CEO of ALSTEM, presented the history of viral gene therapy, from concept formation and early clinical attempts to recent successful clinical applications. He discussed the use of lentivirus as a gene delivery tool and provided details of different generations of the lentivirus packaging systems. Lentiviral approach was recognized as a valuable tool in both ex-vivo and in-vivo clinical applications such as, β-thalassaemia, cerebral adrenoleukodystrophy, and chimeric antigen receptor-T cells.

Dr. Haifeng Chen, CEO of Virovek, Inc., focused on the development of AAV in large scale of manufacturing and process for clinical application. Two major challenges for AAV manufacturing are its productivity and infectivity. Dr. Chen tackled these problems by introducing an artificial intron in the AAV vector and developed a serum free BAC-TO-AAV system with high infectivity and productivity. Under his leadership, Virovek now has the capacity to provide purified AAV vectors exceeding 1 x 10^17 vg scale per production run.

Dr. Niren Murthy, Professor in the Department of Bioengineering at UC Berkeley, shared non-viral technologies and biomaterials developed by his lab for gene delivery. He assembled a delivery vehicle termed CRISPR-Gold that composed of gold nanoparticles complexed with the Cas9/gRNA ribonucleoprotein (RNP), donor DNA, and an endosomal disruptive polymer. The CRISPR-Gold enabled the correction of DNA mutation that causes Duchenne Muscular Dystrophy (DMD) in mdx mice via homologous DNA recombination.
Dr. Kee-Hong Kim, Senior Vice President, Manufacturing & Technical Operations of Tenaya Therapeutics, talked about manufacturing clinical AAVs at scale for gene therapy. He discussed strategic questions for AAV manufacturing, considerations for clinical AAV manufacturing and regulator’s expectation for gene therapy CMC, as well as suggestions for developing a start-up gene therapy company. His suggestions included talent acquisition in AAV manufacturing, compliance of gene therapy CMC, and strategic positioning of products in the market.

Finally, Dr. Shengjiang Liu, Chief Scientist and Head (VP) of Global Pathogen Safety of Product Supply-Biologics of Bayer Pharmaceuticals, discussed the hopes and challenges in gene therapy. He talked about the history of gene therapy, current ongoing over 3,000 clinical trials, global market, as well as regulation and reimbursement issues. He emphasized the challenges of gene therapy such as purification efficiency, and manufacturing capacity but also reminded the audience of all the opportunities when we entered the modern era of gene therapy.

Acknowledgement
We would like to express our gratitude to Dr. Shengjiang Liu, Dr. Gary Li, Dr. Haifeng Chen, Dr. Niren Murthy and Dr. Kee-Hong Kim for their excellent presentations.


We are very thankful for CABS volunteers including Dr. Xiang Yi, Dr. Ken Zhang, Dr. Hao Shao, Ms. Xu Chen, Dr. Hesong Han, Dr. Furong Yu, Dr. Xuefeng Wang, Dr. Qiang Gan, Ms. Ruowei Liu, Dr. Xiangle Sun, and Ms. Jing Chen for their great volunteer works including the event organization and logistics.

You and your family are cordially invited to our Annual Summer Networking Picnics, hosted by CABS Social Life Committee.
BBQ grilled pork, beef, chicken, hot dog, hamburger, salad, soft drinks, fruits, desserts and a lot more!
All you can eat and drink! Plus music and summer breeze!
With a bonus of unlimited FUN and unexpected opportunity for making new friends and social/career interactions!!!
Lucky draws: $25 gift cards and other secret surprises!!!
Online registration is strongly recommended.
Online:
Active CABS member: $10
Non-member: $15
Senior >65 or 5<Child <12: $8
Child < 5: free
Onsite:
Active CABS member: $15
Non-member: $20
Senior >65 or 5<Child <12: $8
Child <5: free
On-line Registration closes on September 6, 12:00 PM (noon).

Sponsorship
New Life Acupuncture
Redwood Picnic Area; 30 Twin Pines Lane, Belmont, CA 94002
Want to join CABS 2020 Executive Council (EC)? Please submit your application here:
http://www.cabsweb.org/ec-election-and-volunteer/
Application deadline: 12AM, August 25, 2019
Only CABS Active Member's application will be considered. Join CABS before submitting your application.
Click here to see what each EC committee is doing: http://www.cabsweb.org/organization/
online
Announcing 2019 CABS K. Fong Award in Life Sciences
The Chinese American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that JOHN V. OYLER is the winner of the 2019 CABS K. Fong Award in Life Sciences for his entrepreneurship and business leadership to establish BeiGene as a world-class biopharmaceutical company. We will honor Mr. Oyler in the award ceremony at the 21st CABS Annual Meeting of the BioPacific Conference to be held on Saturday, June 22, 2019 at the San Mateo Marriott San Francisco Airport Hotel (1770 South Amphlett Blvd., San Mateo, CA 94402).

Mr. John V. Oyler
John V. Oyler is the Chairman, Co-Founder and CEO of BeiGene. BeiGene is a global, commercial-stage biopharmaceutical company focused on the development of molecularly-targeted and immuno-oncology cancer therapeutics. Founded originally as a R&D company in 2010 by Mr. Oyler and renowned scientist Dr. Xiaodong Wang, BeiGene is listed on both the NASDAQ and the Hong Kong Exchange. Under Mr. Oyler’s leadership BeiGene has transformed into a global biopharmaceutical company covering the full biopharma value chain, from R&D and clinical development, to manufacturing and commercial operations. The company currently has three late-stage clinical assets with near-term global commercial opportunities and three marketed cancer products licensed in China. The past nine years has seen tremendous growth at BeiGene; today, the company has 10 offices located in four countries and employs more than 2,200 people across the globe. BeiGene truly epitomizes the ongoing integration of China and the U.S. in science and technology, which resonates with the mission we serve at CABS.
From 2005 to 2009, Mr. Oyler served as President and Chief Executive Officer of BioDuro, LLC, a drug discovery outsourcing company, which was acquired by Pharmaceutical Product Development Inc. in 2010. From 2002 to 2004, Mr. Oyler served as Chief Executive Officer of Galenea Corp., a biopharmaceutical company dedicated to the discovery of novel therapies for central nervous system diseases, which initially were developed at the Massachusetts Institute of Technology. From 1997 to 2002, Mr. Oyler was a Founder and the President of Telephia, Inc., which was sold to The Nielsen Company in 2007. From 1997 to 1998, Mr. Oyler served as Co-Chief Executive Officer of Genta Incorporated (NASDAQ: GNTA), an oncology‑focused biopharmaceutical company. Mr. Oyler began his career as a management consultant at McKinsey & Company. Mr. Oyler received his B.S. from Massachusetts Institute of Technology and MBA from Stanford University.
About CABS K. Fong Award in Life Sciences
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria will be based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or the research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the CABS community and to promoting international collaboration in life sciences.
About Dr. Kenneth Fong
Dr. Kenneth Fong has spent the last 32 years in the biotech industry after completing his academic pursuit in biomedical research.
He is best known for founding the biotech company, Clontech in 1984 where he had cultivated it to be one of the largest biomedical tool companies founded by an Asian American in the US (400 people with 65 PhD scientists). Clontech was sold to Becton Dickinson in 1999 and Ken has continued his career as a Venture capitalist with Kenson Ventures that he founded. He has since cultivated more than 10 highly successful entrepreneurs, advising them and working with them on the growth of their companies.
Currently, he sits on the board of 4 biotech companies and he was intimately involved with the M/A and IPO of more than 10 companies that are worth north of $3 billion. These companies range from research tools, medical diagnostics and drug development. In almost all cases, Dr. Fong has been instrumental in providing strategies for sustainable growth, value creation and liquidity. Those successful entrepreneurs have moved on to assume leadership in other start-up and mid-sized companies, which in turn leads to a new generation of entrepreneurs.
Ken has held a number of leadership positions over the years. He served as the President of the Society of Chinese Bioscientists in North America (2006-07) and President of the Bay Area Asian American Manufacturers' Association (AAMA, 1987). He was also the member of the Board of Trustees of the California State University System (2006-13). His philanthropic interests include scholarships to San Francisco State University, the Kenneth Fong-Hearst endowed scholarships to the CSU system and 40 student scholarships to Peking University. In 2006, he was involved with establishing the Fong Optometry and Medical library at UC Berkeley, and more recently an endowed professorship at Stanford University and a technology translation endowed fund at San Francisco State University.
Ken obtained his PhD from Indiana University and his BS from San Francisco State University.
Past recipients of CABS K. Fong Awards
2018: Dr. Yuling Luo, Founder, CEO and Chairman of Alamar Biosciences, and Dr. Guoliang Yu, Executive Chairman of Crown Bioscience, for their successful serial entrepreneurship in the life science business.
2017: Dr. Yinxiang Wang, Co-founder and CSO of Beta Pharma, for his role in leading development and commercialization of Conmana®, the first small molecule oncology drug specifically targeting cancer cells that is completely developed in China, and Dr. Edgar Engleman, for his pioneering research that was the basis of the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA.
2016: Dr. Gerald Chan, co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, of Stanford University, for his pioneering research in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life- saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth, Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.
online

Instructions for Sponsors
Greetings!
Welcome to 2019 BioPacific Conference!
Please find the information below for setting up the exhibition booth.
Exhibition Hall: Convene lobby and Inspire room #1, #2, #3 (Located at second floor of Marriott)
Exhibition booth includes:
One exhibition table (size: 6’ x 30’’) and two chairs
Exhibition hours: 8:00 am - 7:00 pm
Booth set up time: 7:00 am - 8:00 am
Wrap up time: 6:00 pm - 7:00 pm
Exhibition table assignment:
Please see below for exhibition hall layout and your table assignment.
Notes:
- Please register all the representatives from your company by June 18, 2019 so we can order enough lunch.
- Exhibition hall will be open at 7:00am. Please come on time to set up the booth. The attendee’s registration starts from 8:00am. You can pick up your name badge at the Sponsors’ registration table.
- It is highly recommended to have at least one of your company representatives stay at your booth from 8:00am-5:00pm (you can stay until 7pm, if you like) to introduce your products/services.
- Parking in the hotel parking lot is complimentary so no stamp is needed.
- Free coffee and lunch will be provided.
- All sponsors are welcome to the post-conference reception (5:00pm-7:00pm) to celebrate CABS 21 years of excellence.
Please direct any questions to:
Xu Chen & Frank Hu
Co-chairs, Alliance Management Committee, CABS
E-mail: fundraising@cabsweb.org
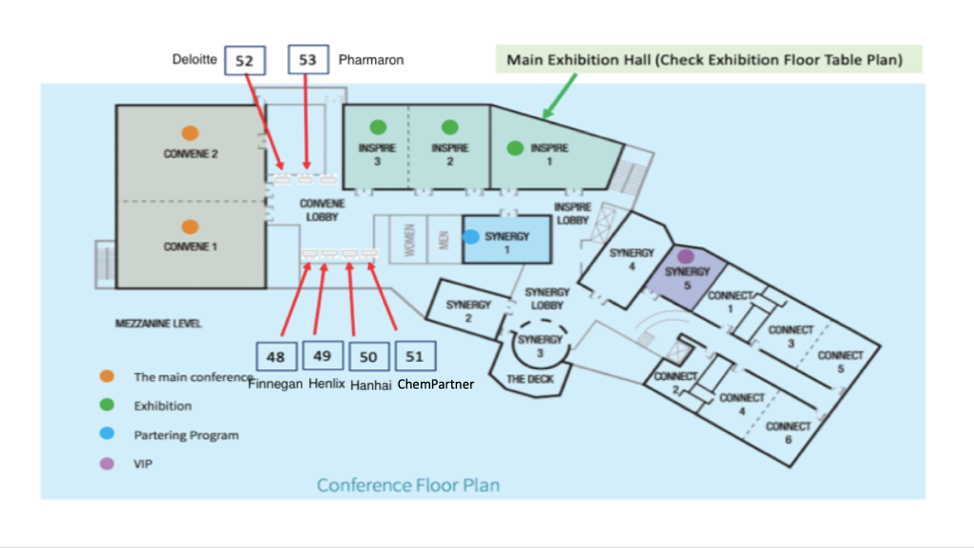

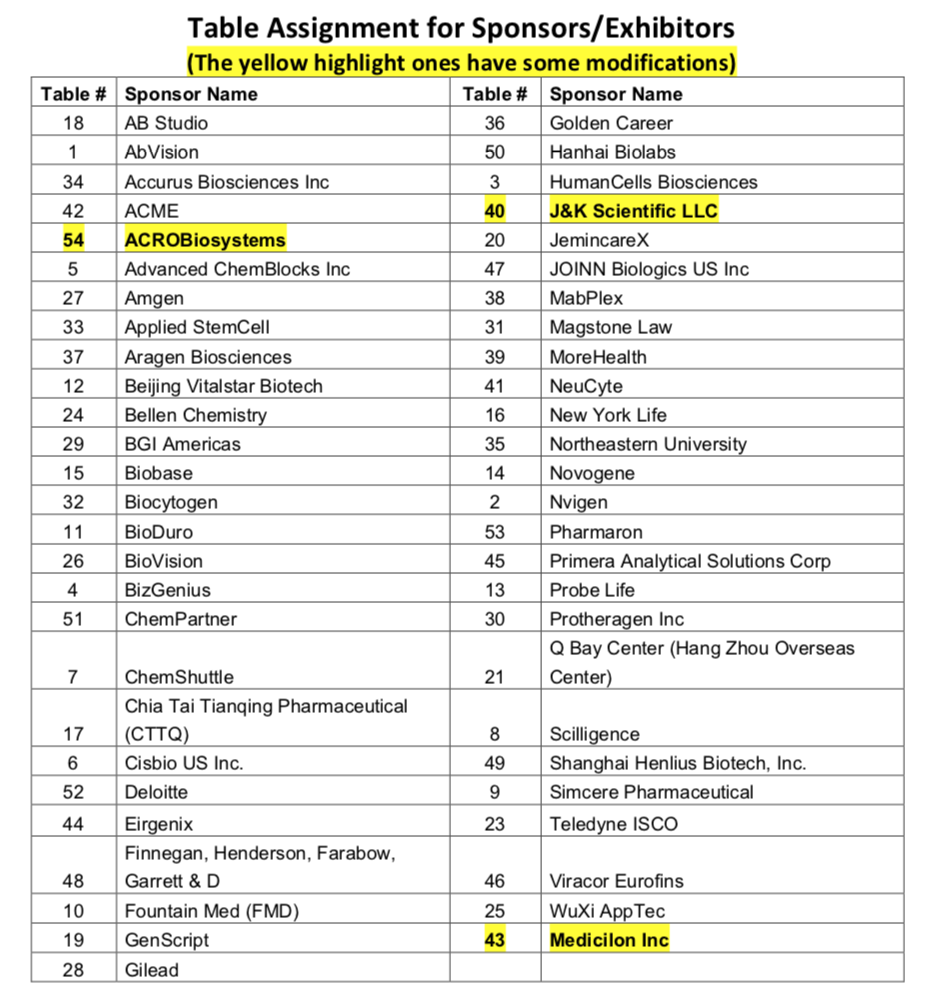
Thanks to our Sponsors!

As of May 27, 2019, all exhibition tables for 2019 BioPacific Conference have been reserved by sponsors. If you have not received invoices from CABS, please do not pay online directly. You are very welcome to contact fundraising@cabsweb.org if you still wish to sponsor CABS without exhibition table. Thank you very much!
San Mateo Marriott San Francisco Airport, 1770 South Amphlett Blvd, San Mateo, CA 94402

Download Conference Full Program Here (PDF)
CABS is one of the largest non-profit biopharmaceutical professional organizations in San Francisco Bay Area. As the flagship event of CABS, BioPacific Conference comes to its 21st anniversary in 2019. This year the conference theme is “Pursuing Science for Cure”. The conference is expected to attract more than 600 attendees from >100 biopharmaceutical companies. This year conference features 8 podium talks presented by industry and academic leaders, covering both science and business aspects of biopharma industry. The conference also features 3 panel discussions covering life science investment, contract services in life sciences and next wave of innovative medicine for cancer.
Will you be interested to know?
- 10 Years growth story of BeiGene
- Denali’s new approaches for neurodegenerative disease
- Discovery story behind Talazoparib: 2018 approved therapeutics for breast cancer
- The recent advances in cancer biomarker and precision medicine
- Next wave of cancer therapies and combinations
- 2019 AACR and ASCO update
- The latest foreign investment regulation and policy
Early Bird Price Ended; Please Do Regular Registration
Scroll down to the bottom to register; or use Eventbrite to register.
Registration using a computer is preferred.
Cannot register over iPhone? Read this or use Eventbrite to register
Please contact info@cabsweb.org, if there are any questions.
Featured Podium Talks
Keynote: Biomarkers and translational research in cancer therapy
Ron Mazumder,PhD, VP, Global Head of Oncology Biomarker,Genentech
Dr.Ron Mazumder has more than 20 years experiences in predictive biomarkers and precision medicines. He has served senior positions at J&J and Merck before joining Genentech in 2016. His talk will focus on predictive biomarker and translational research in immuno-oncology.
Talazoparib: From Virtual Drug Discovery to FDA Approval
Leonard Post, PhD, CSO, Vivace Therapeutics
This is a legendary drug discovery story you cannot miss. Talazoparib, a novel PARP inhibitor, was initially discovered in 2007 by a US-based virtual company Lead Therapeutics, with most of its discovery work done in China. Dr. Leonard Post, CSO of Lead Therapeutics, was involved in its discovery. In 2010, BioMarin acquired Talazoparib for $97M and Dr. Post became the CSO of BioMarin. In 2015, Medivation acquired Tapazoparib for $570M and in 2016, Pfizer acquired Medivation. In 2018, Talazoparib was approved for breast cancer. Journey through Talapzoparib for past 12 years, Dr. Leonard Post will share his first-hand experiences from building a virtual company to FDA approval.
CABS 2019 K.Fong Award Speech- The Future of the Biopharma Industry: Innovation Beyond Science
John V. Oyler, Founder, CEO and Chairman, BeiGene
During a dinner in 2010, John Oyler and renowned scientist Dr. Xiaodong Wang decided to “build China’s Genentech” and thus BeiGene was born. Within a decade, John has led BeiGene to be one of China’s most innovative biotech companies. BeiGene is listed in both NASDAQ and HKSE with market capital more than $7B. What are the stories behind BeiGene’s 10 years growth? What is the current status and future of biopharma industry, both in China and in the world? John will share his perspective during CABS 2019 K. Fong award speech. After the speech, Dr. K. Fong and John Oyler, a legendary venture investor and a legendary entrepreneur, will have a face-to-face chat to discuss more on China’s biopharma industry.
New Approaches To Neurodegenerative Disease Drug Discovery and Development
Zachary Sweeney, PhD, Head of Therapeutic Discovery, Denali Therapeutics
Alzheimer’s disease is one of the most difficult diseases in biopharma drug R&D. From 1998 to 2017, there are >100 failed trials for it. Founded by a group of Ex-Genentech veterans in neurosciences and fueled with record-setting Series A funding, Denali believes it can tackle Alzheimer’s disease differently. What are the unique approaches? Before joining Denali, Dr. Zach Sweeney has been senior leaders in Novartis, Genentech and Roche. He will present an overview of selected development programs of Denali.
Key Developments: CFIUS, Export Control Reform, and Recent Decisions Affecting Life Sciences Investment and Licensing
Joseph Benkert, Senior Advisor, National Security Group, Morrison & Foerster
Last summer the Committee on Foreign Investment in the United States (CFIUS) issued new regulations on the review of inbound foreign investments and export control of outbound licensing. What are the impacts of these new regulations on US – China cross-border investment? How to navigate these new changes? Joseph Benkert is a former US Department of Defense Assistant Secretary of Defense for Global Security Affairs under both the Bush and Obama administrations. He has many years experiences handling complex matters before CFIUS. Joseph has presented the latest CFIUS regulations at CABS 2019 Investor Forum in January 2019. He is back to CABS with new updates on CFIUS regulations since January.
Applying Precision Medicine One Patient at a Time
Shivaani Kummar, MD, Professor of Medicine, Stanford University
With the increasing number of anticancer therapeutic choices, it is possible to tailor treatment options based on individual patients. Professor Shivaani Kummar is an internationally renowned expert in cancer therapy clinical trials, especially in early stage trials. She is currently the Director of the Phase I Clinical Research and Translational Oncology Programs at Stanford University, where she has managed ~20 clinical trials in oncology. In this talk, Professor Kummar will focus on using predictive biomarker and genomic drivers to identify the “right” patients and how to design better early clinical trials that can help making key decisions on later stage trials.
| New Diagnostics Ecosystem to Accelerate Drug Discovery and Medical Innovation |
|
Jason Liu, PhD, MBA, CEO, WuXi Diagnostics The increasing cancer incidence and mortality accelerate demand for targeted drugs and companion diagnostics and economic pressures to develop maximally efficient treatments in China. With the encouragement from the China government, it’s estimated that by 2021, the market size of companion diagnostics will reach 741 million USD, with CAGR of 28%, far exceeding the global scale as 20.1%. WuXi Diagnostics enables precision medicine through a unique dual-enabling platform for in-vitro diagnostic service and product innovation. Our advanced platform integrates multi-dimensional, multi-omics medical data to generate deep clinical insights, and pioneer innovation by building a novel eco-system for healthcare industry. |
Featured Panel Discussion
|
Panel Discussion: innovative therapy in oncology, what is next? |
|
Moderator: Cheni Kwok, PhD, CLP, Managing Partner and Founder, Linear Dreams |
|
Panelists: |
|
Wenfeng Xu, PhD, VP, Henlius |
|
Jean Cui, PhD, CSO, Turning Point Therapeutics |
|
Mark Nevins, MS, VP of Business Development, Apexigen |
|
Peiwen Yu, PhD, VP of Discovery Biology, Exelixis |
|
Tara Arvedson, PhD, Director of Research, Amgen |
| Yanyan Zheng, PhD, Principle Scientist, Merck |
|
|
In this panel discussion, experts from both large biopharma companies such as Amgen and Merck, as well as rising-star oncology startups, will discuss what's next wave of anticancer drugs.
Key Insights from AACR and ASCO 2019
- What was the most exciting data at AACR or ASCO this year?
- What are the emerging hot targets?
Where is the field going in terms of next generation biotherapeutics?
- Antibody formats (ADC, bispecific, multi-specific...)
- New fusion proteins
- Cell therapies (such as CAR T cell, stem cells...)
- Gene therapies (virus-based, modified RNA, anti-sense... )
- Cancer vaccines
What are the future trends for small molecule cancer drugs ?
Conference Agenda (Tentative)
|
Morning Session |
| Keynote: Biomarkers and Translational Research in Cancer Therapy |
| Ron Mazumder, PhD, Vice President and Global Head of Oncology Biomarker Development and Companion Diagnostics, Genentech |
| New Diagnostics Ecosystem to Accelerate Drug Discovery and Medical Innovation |
| Jason Liu, PhD, MBA, CEO, WuXi Diagnostics |
| CABS 2019 K. Fong Award in Life Sciences |
| Presenter: Kenneth Fong, PhD, Chairman, Kenson Ventures |
| Award Speech: The Future of the Biopharma Industry: Innovation Beyond Science |
| Awardee: John Oyler, Founder, CEO and Chairman of BeiGene |
| Designing Therapies to Cure HIV |
| Romas Geleziunas, PhD, Executive Director, Gilead Sciences |
| Fireside Chat |
| Moderator: Alex J. Zhang, PhD, MBA, CEO, Hanhai Silicon Valley |
| Jun Xiang, PhD, General Manager, ChemPartner Biologics |
| Key Developments: CFIUS, Export Control Reform, and Recent Decisions Affecting Life Sciences Investment and Licensing |
| Joseph Benkert, Senior Advisor, Morrison Foerster |
| Panel Discussion: IP, legal and investment in Life Sciences |
| Moderator, Huijun Zhou, FACMG, Founder and CEO, iDNA |
| Panelists: |
|
Joseph Benkert, Senior Advisor, Morrison Foerster |
| Greg Scott, Founder and Chairman, ChinaBio |
| Michael Su, MS, JD, Attorney, Intellectual Property Law, Finnegan |
| Vivien Wang, MS, Partner, Deloitte |
| Lunch Workshop: Humanized Mouse Model Accelerates Biomedicine Research & Drug Development |
| Ming Yin, PhD, VP, Beijing VitalStar Biotechnology |
|
Afternoon Session |
| New Approaches To Neurodegenerative Disease Drug Discovery and Development |
| Zach Sweeney, PhD, Head of Therapeutic Discovery, Denali Therapeutics |
| Panel Discussion: Innovative and Collaborative Contract Services to Accelerate Drug Development |
| Moderator, Jiangwen Majeti, PhD, MBA, Genentech |
| Panelists, |
| Tao He, PhD, Co-Founder and SVP, Joinn Biologics |
| Connie Sun, PhD, SVP, Pharmaron |
| Mike Chen, CEO, AcroBio |
| Applying Precision Medicine One Patient at a Time |
| Shivaani Kummar, MD, Professor of Medicine and Director of Phase I Clinical Research Program (Oncology), Stanford University |
| Panel Discussion: innovative therapy in oncology, what is next? |
| Moderator: Cheni Kwok, PhD, CLP, Managing Partner and Founder, Linear Dreams |
| Panelists, |
| Wenfeng Xu, PhD, VP, Henlius |
| Jean Cui, PhD, CSO, Turning Point Therapeutics |
| Mark Nevins, MS, VP of Business Development, Apexigen |
| Peiwen Yu, PhD, VP of Discovery Biology, Exelixis |
| Tara Arvedson, PhD, Director of Research, Amgen |
| Yanyan Zheng, PhD, Principle Scientist, Merck |
| Talazoparib: From Virtual Drug Discovery to FDA Approval |
| Leonard Post, PhD, CSO, Vivace Therapeutics |
More Details About Our Speakers
Program/Agenda (PDF)
Podium Speakers Bio (PDF)
Discussion Panelists Bio (PDF)
Early Bird Price Ended; Please Do Regular Registration
Scroll down to the bottom to register; or use Eventbrite to register.
Registration using a computer is preferred.
Cannot register over iPhone? Read this or use Eventbrite to register
Please contact info@cabsweb.org, if there are any questions.
Refreshments, lunch and evening reception/happy hour are included.
| CABS Member | Early Bird Registration (by 6/7/2019) | Regular Registration (6/8/2019- 06/21/2019) |
| General | $60 | $100 |
| Academic | $40 Please use your institution email (.edu) |
$80 Please use your institution email (.edu) |
| Non-Member* | Early Bird Registration (by 6/7/2019) | Regular Registration (6/8/20189-06/21/2019) |
| General | $90 | $130 |
| Academic | $70 Please use your institution email (.edu) |
$110 Please use your institution email (.edu) |
* Join CABS today ($30 for one year membership) to save on registration fee: http://www.cabsweb.org/members/
Thanks to our Sponsors!

As of May 27, 2019, all exhibition tables for 2019 BioPacific Conference have been reserved by sponsors. If you have not received invoices from CABS, please do not pay online directly. You are very welcome to contact fundraising@cabsweb.org if you still wish to sponsor CABS without exhibition table. Thank you very much!

San Mateo Marriott San Francisco Airport, 1770 South Amphlett Blvd, San Mateo, CA 94402
Organization: PBSS (Pharmaceutical and BioScience Society) and BioScience Forum
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Venue: Crowne Plaza, Foster City, CA
The 18th Annual San Diego BioPharma Conference & SABPA Bio-Partnering
WHEN
June 8, 2019, 8:00 am - June 8, 2019, 5:00 pm Register
WHERE
Hilton San Diego/Del Mar, 15575 Jimmy Durante Blvd, Del Mar, CA
FEE
Regular Attendees: $45, Full-time Students: $30, Onsite Regular: $80, Onsite Student: $50 (SABPA member will get up to $35 discount)
SUMMARY
San Diego BioPharma Conference & Bio-Partnering is the flagship event of SABPA. This year’s keynote speakers include professor Leiping Chen, one of the pioneers in cancer immunotherapy and Dr. Barry Sharpless, a Nobel Laureate in Chemistry. Multiple heavyweight success stories on recent drug approvals and their real-world impacts will also be showcased. The panel discussion will keep you abreast of the latest trends for cross-border collaboration and investment.
Bio-Partnering is a great platform to explore opportunities in licensing, investment, fund raising and other collaboration deals. It is designed for you to pre-arrange 1-on-1 face-to-face meetings with potential partners/investors/entrepreneurs by using the EBD online tool PartneringOne.
Come and meet some of the visionary leaders and network with your peers. This is one of the biggest San Diego biotech events that you don't want to miss. Please visit http://www.sabpa.org for more details.
https://www.sabpa.org/2019/02/18th-san-diego-biopharma-conference-8th-pacific-bio-partnering/
Hilton San Diego/Del Mar, 15575 Jimmy Durante Blvd, Del Mar, CA
CABS Entrepreneurship Club Workshop
“Biotech Entrepreneurship and Venture Capital “
Agenda:
2:00-2:10pm Opening Remarks: Dr Huijun Zhou, CABS E Club Program Chair
2:10-2:40pm Dr. Alexis Ji, Partner, Illumina Ventures
2:40-3:10pm Dr. William Dai, Founding Partner, ShangBay Capital
3:10-3:40pm Panel Discussion: Dr. Alex Zhang, Alexis Ji, William Dai, Huijun Zhou
3:40-4:00pm Networking
Free Registration is required
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Workshop: CMC Development for Biologics: Purification,Formulation, Analytical Characterization, Production Systems, Regulatory and Product Development
Date/Time: May 6, 2019; 8:45 - 17:00
Venue: Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Intellectual property (IP) rights are critical assets for biotech and pharmaceutical companies. To have a successful exit strategy in your adventure, it is important to consider how to best position and properly protect your IP from the very beginning. What can you learn from recent developments on hot technologies from an IP perspective? How should you prepare for due diligence when raising funds for your startup?
To gain insightful knowledge about the questions above, you are very welcome to attend our legal workshop “Intellectual Property Strategies for Startups: Grow and Exit,” which will be held on April 27, 2019, at Hanhai Biolabs in Burlingame, CA.
Registration is required to attend this workshop. Seats are limited. We sincerely invite you to register as early as possible!
Agenda

Bing Hai is a Senior IP Counsel at Bristol-Myers Squibb Company (BMS), a global
 biopharmaceutical company dedicated to developing innovative medicine for treating serious diseases. She supports discovery and development efforts in various disease areas as well as business transactions such as licensing and M&A from an IP perspective. Her responsibilities include patent filing and prosecution, client counseling, freedom-to-operate analysis, IP due diligence, and agreement negotiation and drafting. She has also provided IP guidance to local commercial teams in various regions including Europe, Latin America, China, and Japan. Prior to BMS, Bing was a patent attorney at Finnegan, where she focused on patent prosecution, IP due diligence and client counseling. Bing earned her Ph.D. in biology at the University of Rochester and had a significant career as a scientist in biotech before receiving her J.D. from UC, Hastings College of the Law.
biopharmaceutical company dedicated to developing innovative medicine for treating serious diseases. She supports discovery and development efforts in various disease areas as well as business transactions such as licensing and M&A from an IP perspective. Her responsibilities include patent filing and prosecution, client counseling, freedom-to-operate analysis, IP due diligence, and agreement negotiation and drafting. She has also provided IP guidance to local commercial teams in various regions including Europe, Latin America, China, and Japan. Prior to BMS, Bing was a patent attorney at Finnegan, where she focused on patent prosecution, IP due diligence and client counseling. Bing earned her Ph.D. in biology at the University of Rochester and had a significant career as a scientist in biotech before receiving her J.D. from UC, Hastings College of the Law.
Michael Liu Su is an attorney based in Finnegan’s Palo Alto office. His practice spans both
 patent and trade secret matters, involving industries such as biotechnology, pharmaceuticals, chemical, medical devices, and computer technology. His litigation experience includes representing clients before state and federal trial and appellate courts, and before arbitrators. He has also successfully litigated before the Patent Trial and Appeal Board (PTAB) in post-grant proceedings that involve patent validity. He also maintains an active patent procurement and due diligence practice. In his graduate work, Mike focused on cancer research at the cellular level, specifically in the areas of cell cycles, cell migration, and cancer metastasis. Mike earned an M.S. in genome sciences from National Yang-Ming University in Taiwan and a J.D. from U.C. Berkeley, School of Law.
patent and trade secret matters, involving industries such as biotechnology, pharmaceuticals, chemical, medical devices, and computer technology. His litigation experience includes representing clients before state and federal trial and appellate courts, and before arbitrators. He has also successfully litigated before the Patent Trial and Appeal Board (PTAB) in post-grant proceedings that involve patent validity. He also maintains an active patent procurement and due diligence practice. In his graduate work, Mike focused on cancer research at the cellular level, specifically in the areas of cell cycles, cell migration, and cancer metastasis. Mike earned an M.S. in genome sciences from National Yang-Ming University in Taiwan and a J.D. from U.C. Berkeley, School of Law.
Yieyie Yang, Ph.D., is an attorney based in Finnegan’s Washington, D.C. office. She
 focuses on complex patent litigation before U.S. district courts and inter partes review (IPR) proceedings in the chemical, pharmaceutical, and biotechnological fields. She has experience representing pharmaceutical patent holders in Hatch-Waxman litigations and has drafted numerous appellant and appellee briefs for pharmaceutical clients. Yieyie also has experience in due diligence analyses of antibodies and their therapeutic uses, vaccine products, immune cell therapy products, and gene therapy products. Yieyie’s scientific background is in molecular and cancer biology. Her graduate research focused on identifying novel genes involved in axon guidance during the development of the nervous system, and her new findings were published in peer-reviewed scientific journals. During her post-doctoral research period at the Dana-Farber Cancer Institute, she studied epigenetic chromatin-remodeling complexes and identified tumor suppressor candidates that interact with oncogenes during cellular transformation and tumorigenesis. Yieyie earned a Ph.D. in molecular biology at the University of Kansas and a J.D. at George Washington University Law School.
focuses on complex patent litigation before U.S. district courts and inter partes review (IPR) proceedings in the chemical, pharmaceutical, and biotechnological fields. She has experience representing pharmaceutical patent holders in Hatch-Waxman litigations and has drafted numerous appellant and appellee briefs for pharmaceutical clients. Yieyie also has experience in due diligence analyses of antibodies and their therapeutic uses, vaccine products, immune cell therapy products, and gene therapy products. Yieyie’s scientific background is in molecular and cancer biology. Her graduate research focused on identifying novel genes involved in axon guidance during the development of the nervous system, and her new findings were published in peer-reviewed scientific journals. During her post-doctoral research period at the Dana-Farber Cancer Institute, she studied epigenetic chromatin-remodeling complexes and identified tumor suppressor candidates that interact with oncogenes during cellular transformation and tumorigenesis. Yieyie earned a Ph.D. in molecular biology at the University of Kansas and a J.D. at George Washington University Law School.
Gavin Liu is an associate in the Silicon Valley office of Latham & Watkins.  Mr. Liu’s practice focuses on commercial and intellectual property transactions for public and emerging life science companies on a wide variety of transactions including collaboration and strategic alliance agreements, licensing arrangements, joint ventures, manufacturing, supply, and distribution agreements, M&A transactions, capital markets transactions, and strategic counseling. Most recently in March of 2019, Gavin represented Daiichi Sankyo in a multi-billion dollar global development and commercialization agreement with AstraZeneca for Daiichi Sankyo’s lead antibody drug conjugate product [fam-] trastuzumab deruxtecan.
Mr. Liu’s practice focuses on commercial and intellectual property transactions for public and emerging life science companies on a wide variety of transactions including collaboration and strategic alliance agreements, licensing arrangements, joint ventures, manufacturing, supply, and distribution agreements, M&A transactions, capital markets transactions, and strategic counseling. Most recently in March of 2019, Gavin represented Daiichi Sankyo in a multi-billion dollar global development and commercialization agreement with AstraZeneca for Daiichi Sankyo’s lead antibody drug conjugate product [fam-] trastuzumab deruxtecan.
Gavin received his JD in 2014 from the University of California, Berkeley School of Law; during his time in law school, he served on the Editorial Board of the Berkeley Technology and Law Journal and is an alumni of the Samuelson Law, Technology, and Public Policy Clinic. Prior to law school, Gavin received his B.S. in Neuroscience from Duke University and worked in the labs of Allison Doupe and Michael Brainard at the Keck Center for Integrative Neuroscience at the University of California, San Francisco.
Hanhai BioLabs Inc., 1633 Old Bayshore Highway, Suite 280, Burlingame, CA 4010
Workshop: Tox-21 and New Approach Methodology (NAMs): Using Emerging Technologies to Improve Translational Research and Safety Assessment Predictions
Date/Time: April 25, 2019; 8:30 - 17:00
Venue: South San Francisco Conference Center, SSF, CA
Organization: NorCal-SOT & PBSS
Details& Registration: https://protect2.fireeye.com/url?k=63ab6dad-3feb5893-63ab4ab0-0cc47ad9c120-6a76bd1827aa2e76&u=https://www.pbss.org/aspx/homeSF.aspx
South San Francisco Conference Center, SSF, CA
CABS Symposium: CAR-T and Cell Therapy
Do you like to be informed of the latest research and development on T cell gene editing and engineering technology? Are you aware of the assay options to detect CAR expression? Do you have any interest in the PK/PD of CAR-T therapy? If the answer is “yes”, you are very welcome to join us in a symposium on CAR-T and Cell Therapy that will be held on April 6, 2019 by CABS Science & Technology Committee. You will also have opportunities to network with some of the industry professionals of T cell immunotherapy.
Date: April 6th, Saturday, 1:00-6:00 PM.
Location: Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010).
Agenda:
1:00 to 1:20 PM Check-in and networking.
1:20 to 1:30 PM Welcome remarks from CABS.
1:30 to 2:15 PM Gene editing in T-cell therapy. Ling-Jie Kong, PhD, Vice President, Technology Development, Applied StemCell, Inc.
Abstract: Cancer is one of the leading causes of death worldwide. Patient derived chimeric antigen receptor-T (CAR-T) cells are a powerful tool in achieving a complete remission in a range of B-cell malignancies, most notably B-acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL). However, there are limitations. First, the manufacture CAR-T cells from the patient’s own T cells is often challenging and costly. Second, the long process often delays the treatment of the patients. Third, the function of T cell from patients usually are compromised which leads to low response rate in some of the patients. Healthy donor, gene-edited CAR-T cells which do not require human leucocyte antigen (HLA) matching have the potential to provide an ‘off the shelf’ product, overcoming the manufacturing difficulties of producing CAR-T cells for each individual patient. Here we will present our work towards this goal using iPSc and gene editing technology, focus mainly on our TARGATT technology on gene knock-In.
2:15 to 3:00 PM An Ideal Solution for the Detection of CAR Expression. Jane Liu, Product Manager, ACROBiosystems.
Abstract: Evaluating CAR expression is an essential step in the production of CAR-T cells. This is often done by flow cytometry, using protein L, anti-Fab antibodies or target antigens as detection antibodies. However, which reagent would be the best choice to detect CAR expression? Here we would like to share some data to show the comparison between different methods and reagents for detecting the CAR expression.
3:00 to 3:15 PM Break
3:15 to 4:00 PM Pharmacokinetic/Pharmacodynamic Aspects of Developing CAR-T Therapies. James Kalabus, PhD, Principal Scientist, Clinical Pharmacology, Modeling & Simulation, Amgen.
Abstract: Chimeric Antigen Receptor (CAR) T cell therapy is a promising new modality in the immuno-oncology arsenal. CAR-Ts are a dynamic “living” biologic that can expand and differentiate during manufacturing and after administration to patients. The pharmacokinetics and pharmacodynamics of CAR-Ts are still relatively uncharted water. Topics covered in this talk will include relevant background, emerging information regarding dose-exposure and exposure-response relationships associated with CAR-T therapy as well as discussion of some of the primary pharmacodynamic and safety concerns related to administration of CAR-Ts.
4:00 to 4:45 PM A Novel T-cell Engineering Technology (ARTEMISTM) with Favorable Efficacy/Safety Profile in Solid and Hematologic Cancers.Jingyi Xiang, PhD, Director of Scientific Alliances, Eureka Therapeutics, Inc.
Abstract: Eureka’s proprietary ARTEMIS™ antibody TCR (AbTCR) T-cell receptor platform was designed to create potentially safer and more effective T-cell therapies. In preclinical and clinical studies against CD19-positive malignancies, Eureka’s ARTEMIS™ T-cells matched the cancer killing potency of CAR-T therapies but with a dramatic reduction in the levels of inflammatory cytokines released. Utilizing ARTEMISTM platform together with a proprietary human TCR-mimic (TCRm) antibody to target an AFP-peptide/HLA-A2 complex on HCC cancer cells, ET140202 T-cell therapy demonstrated a favorable safety profile and promising clinical efficacy in preclinical POC clinical studies.
4:45 to 5:30 PM Allogeneic CAR T for Cancer Treatment. Barbra Sasu, PhD, Chief Scientific Officer, Allogene.
Abstract: Chimeric antigen receptors (CARs) consist of antibody fragments recognizing cancer antigens fused to T cell signaling components. When introduced into a patient’s own T cells (autologous CAR T’s), these molecules are designed to mediate fast and efficient recognition and killing of cancer cells expressing the target of interest. Autologous CAR T against hematological targets have shown significant efficacy in the clinic, demonstrating high complete response rates with approximately half the patients who respond to therapy achieving durable long-term remission. This data has led to commercialization of the first two autologous anti-CD19 CAR therapies, Kymriah™ and Yescarta™. Autologous CAR T therapy, while it can be effective, has some limitations. Efficacy of the CAR T product can be impacted by the behavior of the patient cells, with prior lines of treatment and sometimes the nature of the disease itself, impacting the health, proliferative capacity and cytotoxic activity of the cells. This, along with the logistics of patient cell collection and the length of time required to make the cells may impact which patients are eligible for therapy based on disease progression. Finally, every patient’s cells are manufactured individually, which increases the overall cost and complexity of manufacturing. Allogeneic CAR T therapy is the process of making CAR T cells from T cells harvested from healthy donors. These cells are modified to eliminate the TCR which inhibits the ability of an infused cell to recognize the host as foreign, thereby meaning that the only thing the allogeneic cells are designed to recognize is the cancer cells they are intended to target. While allogeneic CAR T research is at an earlier stage, encouraging Phase 1 clinical data demonstrates the promise of this therapy for more patients. The talk will highlight the clinical data available to date and the overall research strategy to develop a pipeline of allogeneic CAR T therapies across a range of hematological and solid tumor indications.
5:30 to 6:00 PM Refreshment & Networking.
Speakers Background:

Dr. Ling-Jie Kong has over 10 years’ experience in drug discovery in the areas of oncology, hematology, metabolic diseases, and gene and cell therapy. He received his PhD in biochemistry from the North Carolina State University, and his post-doctoral training in cancer biology from Dr. Joseph Nevins’ lab at the Howard Hughes Medical Institute, Duke University Medical Center. In 2007, he joined Merck, where he had led several successful drug discovery programs in oncology and metabolic diseases. Dr. Kong joined Applied StemCell as head of its research and technology development team. With his deep interest in drug discovery, gene therapy and passion to bring the best therapeutic strategies to patients, Dr. Kong has spearheaded new discoveries and development in stem cell, bioprocessing and genome editing technologies. Under his guidance, the company has successfully launched several preclinical and IND stage gene therapy programs as well as bioproduction and protein screening platforms.

Jane Liu, Product Manager of ACROBiosystems. She uniquely designed the product line for detecting CAR expression to meet the application needs of CAR-T therapy. She has rich experience in the development and production of CAR-T target protein, fluorescent-labeled protein and pre-biotinylated protein product lines. Jane has extensive communication with global customers and has in-depth experience in system construction of CAR-positive T cells detection. Jane graduated from Jilin University with Master degree in animal breeding & genetics.

James Kalabus, PhD.is a Principal Scientist in the Clinical Pharmacology, Modeling & Simulation group at Amgen in South San Francisco. He currently supports the clinical development of a number of immune-oncology programs including one CAR T candidate. Prior to his work at Amgen, James held positions of increasing responsibility as a Clinical Pharmacologist at Novartis Pharmaceuticals in East Hanover, NJ. James received his PhD in Pharmaceutical Sciences at the State University of New York at Buffalo where he also conducted postdoctoral research in the Pharmaceutical Genetics Laboratory.

Jingyi Xiang, PhD, Director of Scientific Alliances, Eureka Therapeutics, Inc. Dr. Jingyi (Gene) Xiang has over 14 years of experience in biomedical research, with in-depth knowledge and expertise in therapeutic antibody and cell therapy. He has led a team responsible for the detailed biochemical and biophysical characterization of more than a dozen therapeutic monoclonal antibody candidates. He has also been involved with the development of novel T-cell immunotherapy targeting intracellular cancer antigens. Now Dr. Xiang serves as the Director of Scientific Alliances, facilitating the scientific and clinical collaborations with academic and industrial partners.
Dr. Xiang received his B.S. in Chemistry from Peking University, PhD in Biochemistry from the State University of New York at Stony Brook, and completed his postdoctoral training at UC Berkeley.

Barbra Sasu, Ph.D., is the Chief Scientific Officer of Allogene. Barbra joined Allogene as Chief Scientific Officer in April 2018 when the company acquired the allogeneic cell therapy assets from Pfizer. Barbra received a Ph.D. from the Paterson Institute for Cancer Research in Manchester, UK, in the laboratory of T. Michael Dexter, FRS. She conducted postdoctoral research at Harvard Medical School in the laboratory of T. Keith Blackwell, M.D., Ph.D. Barbra joined Amgen in 2001 to work on normal and malignant hematology initially and later expanded her focus to include immune-oncology with an emphasis on T cell redirective therapies, specifically BiTE molecules. In 2013, she joined Pfizer to become involved with large molecule immuno-oncology including the Pfizer T cell redirection programs and led diligence to look for a CAR T collaboration partner. This collaboration search resulted in the Cellectis partnership in 2014 and the collaboration with Servier for clinical execution of UCART19 and other preclinical assets.
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010).
With the theme of ‘Frontiers in Life Sciences’, the 2nd UCSF-ACSS 'Golden Gate' Life Sciences Forum will cover current research in aging, digital health, stroke, immunology, cell therapy, cancer research, et al. We want to establish a bridge between science and industry.
The forum will be divided in to three sections: Experts Forum, Frontiers in Biotechnology, and Young Scholars Forum. It will take place on 30th March, 2019 (Saturday) at the Cole Hall Auditorium, 513 Parnassus Ave, San Francisco, 94143. The forum will formally start at 10.00 am and last for whole day. Scholars, researchers, academic students from UCSF, Stanford, UC Berkeley, USF, State university, AAR , CCA and other universities will come to join us. Last year, our forum attracted around 300 audience. Everyone interested in life science is welcome to join us.
Time:9:30 am to 6:30 pm, March 30th, Saturday
Venue:Cole Hall Auditorium, 513 Parnassus Ave, SanFrancisco, 94143
Price: Free,inc lunch
To register (scan the QR code below or click 'read more' to open the website directly)
https://www.eventbrite.com/e/the-2nd-golden-gate-life-science-forum-tickets-58811591052
Cole Hall Auditorium, 513 Parnassus Ave, SanFrancisco, 94143
Location Change: 2019 CABS|CPGS|ACSS UCSF|BCSSA|CGPSA Bay Area Career Fair
The Chinese-American Bio/Pharmaceutical Society (CABS), Association of Chinese Students and Scholars at UCSF (ACSS UCSF), Berkeley Chinese Students and Scholars Association (BCSSA), and Chinese Graduate and Postdoctoral Scholars Association (CGPSA) at UC Berkeley are hosting a Career Fair on March 17th (Sun), 2019. Please join us if you are interested in:
-
Seeking industrial and academic full-time or internship opportunities in the Bay Area and China
-
Identifying your transferable skills and developing your own career path
-
Boosting your professional networks and meeting potential referrals
-
One-to-one conversation with industrial representatives
When: March 17th (Sunday), 12:30 pm - 5:00 pm
Where: HanHai Biolabs, Suite 280, 1633 Bayshore Highway, Burlingame, CA 94010
Weekend Free Parking.
Agenda:
12:30-1:00 pm Check-in & booth set-up
1:00-1:10 pm Welcome speech
1:10-2:00 pm Company presentations
2:00-4:30 pm Career fair
4:30-4:35 pm Closing remarks
4:35-5:00 pm Venue cleaning up
Attending Companies (in alphabetical order):
-
AbVision, Inc. (AVI), previously the Drug Discovery Group of BioVision Inc., is a privately held biopharmaceutical company headquartered in the San Francisco Bay Area. AVI’s most advanced drug discovery efforts aim to develop pipelines of cancer and infectious disease therapeutics that harness the natural power of the immune system to bring first-in-class and best-in-class drugs to the world.
-
Alibaba Group is a Chinese multinational conglomerate specializing in e-commerce, retail, Internet and technology. Founded April 4, 1999, the company provides consumer-to-consumer (C2C), business-to-consumer (B2C), and business-to-business (B2B) sales services via web portals, as well as electronic payment services, shopping search engines and cloud computing services.
-
Amgen is one of the world’s leading biotechnology companies. Amgen is a values-based company, deeply rooted in science and innovation to transform new ideas and discoveries into medicines for patients with serious illnesses.
-
Ancestry.com LLC is the largest for-profit genealogy company in the world, and it operates a network of genealogical, historical record and genetic genealogy websites.
-
Apostle Inc. is a biotechnology start-up headquartered in Sunnyvale, CA. We are in the business of the research, development, licensing, and sales of novel AI (Artificial Intelligence)-Enabled Nanotechnologies and the related intellectual properties, products, and services for diagnosis and treatment of human diseases.
-
Applied StemCell Inc. (ASC) is a fast-growing biotechnology company providing animal and cell line models as tools for drug discovery and diagnostics. Using our proprietary TARGATT™ gene modification technology, we make site-specific Knockin mouse, rat and human cell line models fast and efficient for pharmaceutical and academic community.
-
AVIDA Biomed develops novel genomic tools to reveal DNA alterations with unprecedented accuracy for clinical and research use. Our team is passionate, experienced and looking to expand.
-
Beijing Genomics Institute (BGI) was established in 1999 with participation in the original Human Genome Project. Now as one of the world’s largest genomics organisations, BGI is experienced in genomic research, commercial NGS services and sequencer manufacturing. We are committed to providing solutions to address the research, pharmaceutical, and clinical markets.
-
Carmot Therapeutics is pioneering the discovery and development of innovative drugs for the treatment of metabolic diseases, cancer, and inflammation. Carmot’s vision is to become a leader in drug discovery by generating superior drugs for challenging therapeutic targets.
-
Gan & Lee Pharmaceuticals is a high-tech biopharmaceutical company specialized in research, development, production and commercialization of insulin and its analogues, leading in the diabetes market in China.
-
Gui Guo Quan is a dedicated job-hunting platform designed for oversea Chinese talents. With the help of Big-Data algorithms, you can find the job position that suits you the most. We also offer the service of online interviewing, online recruiting seminars, and other one-stop career services. With our help, you can find the most suitable job position with minimal effort, and in no time.
-
Hengenix Biotech (Hengenix) focuses on delivering safe and effective yet affordable medicines to treat a range of chronic and life-threatening diseases. Affiliated to Shanghai Henlius Biotech, a top biopharma company in China, Hengenix has a successful track record in moving multiple research leads into clinical stage with a rapid speed, including the first biosimilar approval in China, 2 NDA filings and 27 IND approval in 9 years.
-
IntelliPro Group Inc. is one of the fastest growing integrated talent solution providers in Americas & APAC. We provide comprehensive HR services ranging from Executive Search, Recruiting, IT Consulting, Software Outsourcing, Business Process Outsourcing, Payroll Admin, Compensation and Benefits Consulting, Campus Recruiting, to Technical Training etc. to world-class leading companies such as Google, LinkedIn, Samsung, Pinterest, Adobe, Alibaba, Tencent, etc..
-
Ivy Elite Inc., founded in 2012 in the center of education and economics in the United States - New York City, is uniquely endowed with educational resources in the United States and the most up-to-date information on studying abroad for international students. Ivy Elite Inc., who is praised by the industry as “The Wall Street's Emboldened High-end Study-abroad founder”, has been working hard on this road to creating the most focused, professional and dedicated high-end study-abroad brand.
-
Kelun Pharmaceutical 科伦药业 is the market leader of injectable and infusion technologies. We are committed to R&D for small-molecule NCEs and biological NBEs, biosimilars, generic drugs, and new drug delivery systems (NDDS) in oncology, diabetes, hepatitis, cardiovascular and other major disease areas.
-
MabPlex provides global CDMO services in the development and manufacturing of biopharmaceuticals, including mAbs, recombinant proteins, ADCs and bispecifics. We offer our partners contract services from DNA to finished drug product. Our technical expertise allows us to provide effective high quality solutions for our partners biomanufacturing needs.
-
MedAbome, Inc. is a biotech company located in San Francisco Bay Area that is focused on the discovery and development of innovative therapeutic monoclonal antibodies (mAbs) and immunotherapy technologies for the treatment of major human diseases such as cancers, cardiovascular diseases and autoimmune diseases.
-
MORE Health’s mission is to offer you and your family access to the best medical minds in the world, exactly at the moment you need their experience; when you are faced with decisions regarding a serious, life-changing illness.
-
Morrison & Foerster is an international law firm with 16 offices located throughout the United States, Asia, and Europe. The firm has over 1,000 lawyers who advise clients across a range of industries and practices, including intellectual property, patent litigation, corporate/M&A, business restructuring, and securities
-
New York Life Insurance Company (NYLIC) is the third-largest life insurance company in the United States, the largest mutual life insurance company in the United States.
-
NVIGEN is a nanotechnology empowered personalized medicine innovator. We are focusing on the development of a comprehensive circulating bio-marker biopsy technology, Nanopsy™, to predict cancer recurrence at the earliest possible time and guide the most effective personalized therapy.
-
Paragon Genomics develops and commercializes rapid, easy-to-use, ultra-high multiplex PCR-based target enrichment solutions for Next-Generation Sequencing (NGS).
-
Predicine is a molecular insights company that is committed to developing the best-in-class precision diagnostics and precision medicine portfolio to address the unmet medical needs worldwide.
-
Senti Biosciences is a team of bioengineers, scientists, and entrepreneurs on a mission to treat the most complex and challenging diseases. Led by pioneers in synthetic biology and computation, Senti is using the latest techniques in the fields to build the future of gene and cell-based therapies.
-
Twist Bioscience works in service of customers who are changing the world for the better. In fields such as medicine, agriculture, industrial chemicals and data storage, by using our synthetic DNA tools, our customers are developing ways to better lives and improve the sustainability of the planet.
-
University of Electronic Science and Technology of China 电子科技大学 is a national key university directly under the Ministry of Education of China. UESTC was included as one of the first universities into "Project 211" in 1997, and then the nation's “Project 985” in 2001.
-
Visa Research aims at complementing an already substantial investment in technology development with a focus on the scientific foundations of existing, emerging, and future commerce related technologies across broad time horizons. With the establishment of a formal research organization and the associated long-term commitment to technology research, Visa joins the ranks of a small number of industry leaders with both the insight and ability to substantially influence and systemically impact the future.
-
Wuxi AppTec, established in December 2000, is a leading global pharmaceutical, biopharmaceutical, and medical device open-access capability and technology platform with global operations. Our services are designed to help customers shorten the discovery and development time, lower the cost of drug and medical device R&D through cost-effective and efficient solutions.
-
XMotors is a fully-owned subsidiary of XPENG Motors, developer of the electric vehicle with cutting edge AI features. XPENG Motors is dedicated to applying new technologies, crafts and business models to create AI cars liked by young people, and to refresh user's experience.
And more to come!
All are welcome! We’re looking forward to seeing you on March 17th!
Registration link: https://www.eventbrite.com/e/2019-cabscpgsacss-ucsfbcssacgpsa-bay-area-career-fair-tickets-58556808992
-
HanHai Biolabs, Suite 280, 1633 Bayshore Highway, Burlingame, CA 94010
Economic Update & New Tax Planning Seminar
You are invited to join us for a new tax and transnational Planning seminar, where you can learn some of the key issues to help you plan, protect, and transfer your assets more efficiently.
You will learn useful information on:
1. New concepts in transnational planning in current economic environment.
2. Estate, gift, and income taxations.
3. Common mistakes in family trust planning.
4. Qualifying Domestic Trust.
5. Transnational planning case analysis.
6. Personal and business income tax – related issues
7. Company structures and taxation.
8. New tax planning strategies in real estate business.
Seminar host by CABS. Seats are limited, please RSVP.
Speaker: Aaron Yen, JD. Esq.
Aaron Yen is a California and Nevada attorney who primary focus on the practice areas of domestic and international business and transaction structuring, general counsel, trademark, asset protection, tax and estate planning, tax reduction, trust and succession strategies for business owners and high net worth clients.
Speaker: Winny Lin, MBA, LUTCF, Financial Planner
Winny Lin is a well trained professional financial planner who can help you identify your financial needs and then determine which insurance and financial products can best help you meet your objectives. She brings a wealth of experience and knowledge in providing her clients with proper financial planning and investment advisory services, specializes in retirement, transnational and estate planning. She helps lots of high net worth clients solve the headache problems on wealth protection and transfer planning.
Speaker: Min Zhao, CPA, Master in Taxation
Min Zhao is a member of American Institute of Certified Public Accountant, and is a member of AICPA. She brings a wealth of experience and knowledge in providing her clients with proper tax planning and tax consultation services. She specializes in international tax, corporation structure and taxation, and high net worth clients’ tax strategies. The business areas includes real estate, manufacture, high tech company.
HanHai Biolabs, 1633 Bayshore Highways, suite280, Burlingame, CA 94010
Workshop: Clinical Trials Essentials: An Intensive One-Week Course (register for the full week or half day sessions)
Date/Time: March 11- 15, 2019; 8:30 - 17:00
Venue: 3175 Bowers St., Santa Clara, CA
Organization: UCSC Extension and PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
3175 Bowers St., Santa Clara, CA
Symposium: Promising Therapeutic Approaches and Translational Medicine to Advance Novel Therapeutics (A Joint Symposium by PBSS and the Stanford SPARK Program)
Date/Time: March 7, 2019; 8:45 - 17:00
Venue: Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Dear CABS members,
CABS social life committee is planning the first PingPong activity on March 3, 2019. Please mark your calendar and get ready!
Come out, get active, and play friendly games with our CABS members in spring of the year! We encourage table tennis enthusiasts in all levels to participate.

Location: PongPlanet Table Tennis Club,
Address: 848 Brittan Ave, San Carlos, CA 94070
Date: Sunday, March 3rd, 2019
Time: 10am to 3 pm
Lunch (pizza, drinks, fruits and snacks) will be provided.
*Finalists will be rewarded with gift cards (1st place: $75; 2nd place: $50; 3rd place: $25)
The detail rules are below:
- Two brackets: Men, Women (tentatively, could be mixed genders)
- Rules: Double Elimination or Single Elimination to be determined.
- Match Format: Play in best 3 of 5 games. 11 points per game.
- Balls: Committee will provide 40+ butterfly plastic balls exclusively for this tournament.
- Target Maximum enrollment for each bracket: 20 single players.
- Referees are optional but we should play fair games regardless.
If you interested, please sign in with your name/gender below.
848 Brittan Ave, San Carlos, CA 94070
2019 Enmore Bio Conference
Enmore Bio Conference (EBC) is organized by Enmore Healthcare, with thousands of participants 3/1/2019 to 3/3/2019 each year after Spring Festival in Jiangsu province.
EBC will focus on market investment, precision medicine, meanwhile, integrating the relevant policy direction, cell therapy, antibody drugs and new drug development, molecular diagnostics, development of techniques and clinical application into those topics.
EBC will serve as an international exchange platform for decision makers of related enterprises and promote the innovation cooperation of the healthcare industry.
Date/Time:March 1, 2019 - March 3, 2019
Venue:Nanjing,Jiangsu province,China
Organization: Enmore Healthcare
Official website: http://ebc.enmorebiz.com/
Nanjing,Jiangsu province,China
Dear everyone,
We would like to sincerely invite you to participate in the 2019 Career Advisory Network (CAN) Program as a mentor or mentee. CAN aims to cultivate members to be the next generation of healthcare/life sciences professionals and leaders through mentoring, skill building, and networking. It is a 6-month program with 1:1, small group mentoring meetings, or large group networking sessions. Mentors and mentees are expected to meet once monthly during the months of May through Oct 2019 (in person or virtually).
Matching will be based on a variety of factors such as background, mutual interest, locations. We will officially launch the 2019 CAN program in April (Kick-off meeting date TBD). Here is a tentative schedule for this year’s program:
- Mentor/Mentee registration opens: Friday, 2/15/2019
- Registration closes: Monday, 3/18/2019
- Program acceptance, email notification sent: 1st week of April
- Kick off event: Late April
- Mentoring groups convene: May-October
- Graduation: November
Please visit http://www.cabsweb.org/can/ to learn more about the CAN program. If you are interested in becoming a mentor or mentee, please complete the forms below by midnight on Monday, 3/18/2019.
-
MENTOR registration:
-
MENTEE application:
If you have any questions, please contact the CAN Co-Director Joe Zhang, email address: zzha158@gmail.com.
Best regards,
Joe Zhang
CAN Co-Director
Below are CABS Operational SOPs:
1. Organizing event at Hanhai Lab
How to organize CABS event at Hanhai lab SOP V3.doc
2. BioPacific Conference Registration Logistics
Registration and Logistics Preparation for BioPacific Conference SOP V1.docx
CABS business cards SOP v1.docx
000CABS Business Card_2018 -TEMPLATE 1.pptx
000CABS Business Card_2018-with QR code TEMPLATE 2.pptx
4. Reimbersement
CABS Reimbersement SOP V1.docx
Reimbursement_Request_from.xlsx
5. CABS Equipment
Online
Our website registration system is not stable. If you cannot register over iPhone, please try Eventbrite free registration HERE. Or try again on a PC computer with Chrome browser (IE browser not working here). If still cannot register, please email info@cabsweb.org
Please note that our agenda has been updated below.
Agenda:
1:00 to 1:20 PM Check-in and networking.1:20 to 1:30 PM Welcome remarks from CABS.
1:30 to 2:15 PM Application of Computational Tools in Structural Chemistry: A Retrospective Study of Lead Optimization of Potent Macrocyclic Cyclophilin Inhibitors for HCV. Devleena Shivakumar, PhD, Senior Scientist, Structural Chemistry, Gilead Sciences Inc.
2:15 to 3:00 PM Advances in Enzyme Engineering: Application to the Discovery and Efficient Synthesis of Pharmaceuticals. Jim Lalonde, PhD, Senior Vice President, Research and Development, Codexis.
3:00 to 3:15 PM Coffee Break
3:15 to 4:00 PM Reversal of Adenosine-Mediated Immunosuppression: Small Molecule Inhibitors of CD73. Brandon Rosen, PhD, Senior Scientist, Medicinal Chemistry, Arcus Biosciences.
4:00 to 4:45 PM Curing Hepatitis C: Ledipasvir and Velpatasvir, the NS5A Inhibitors in Harvoni® and Epclusa® John O. Link, PhD, Vice President, Medicinal Chemistry,Gilead Sciences Inc.
4:45 to 5:30 PM Refreshment and networking
Speakers Profile:
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010
WuXi AppTec Workshop 2019 - Discovery, IND and Beyond
WuXi AppTec Lab Testing Division offers the knowledge, scalability and dedicated global resources to successfully
Schedule One-on-One counseling with WuXi AppTec Subject Matter Experts (Discovery, DMPK, PD, CMC, Bioanalytical, TOX, Clinical)
9:00 AM – 10:00 AM Registration/Breakfast
10:00 AM - 10:15 AM
Opening remarks (Ed Amat, VP, International Sales and Marketing, Laboratory Testing Division, WuXi AppTec)
10:15 AM - 10:45 AM
A Roadmap for a Successful IND Program (Yi Jin, PhD, DABT, VP, Chief Scientific Toxicologist & Head of WIND Project Management Laboratory Testing Division, WuXi AppTec)
10:45 AM - 11:15 AM
Summary of Discovery Phase Outcomes (Qunsheng Ji, PhD, VP, Oncology, Research Service Division, WuXi AppTec)
11:15 AM - 11:30 AM Morning Break
11:30 AM - 12:15 PM
Keynote Guest Speaker Presentation
Talazoparib : The development of a PARP inhibitor (Len Post, PhD, CSO, Vivace Therapeutics)
12:15 PM – 1:15 PM Lunch Break
1:15 PM – 2:00 PM
Keynote Guest Speaker Presentation
Nonclinical Strategies for Successful US FDA IND Submissions (Grace Furman, PhD, DABT, President & CEO, Paracelsus)
2:00 PM – 2:30 PM
ADME and PK Attributes for Drug Discovery and Development (Sharon Tong, PhD, Executive Director of DMPK, Laboratory Testing Division, WuXi AppTec)
2:30 PM – 3:00 PM
WuXi’s Services for Drug Safety Assessment (Mark Walker, Sr. Technical Director of Toxicology, Laboratory Testing Division, WuXi AppTec)
3:00 PM – 3:30 PM
Clinical Development Considerations (Bill Taaffe, Corporate Development Officer, WuXi Clinical)
3:30 PM – 3:45 PM Coffee Break
3:45 PM – 4:15 PM
Large Molecule Session - DNA to IND and Beyond: A Comprehensive & Integrated Biologic CMC Roadmap (Sherry Gu, PhD, VP, WuXi Biologics)
4:15 PM – 4:45 PM
Large Molecule Session- Bioanalytical Strategies for Large Molecule: Preclinical Studies and First-in-Human Trial (John Pirro, Sr. Director, Head of New Jersey Bioanalytical Services, Laboratory Testing Division, WuXi AppTec)
4:45 PM – 5:45 PM
Panel Discussion
Rostam Namdari, Ph.D., Senior Director, Translational Drug Development, Xenon Pharmaceuticals Inc.
Edgar Tenorio, DVM. MPVM, Nonclinical Consultant
Ronan Le Moigne, Ph.D., Senior Director, Preclinical Development and Translational Medicine, ESSA
5:45 PM – 5:50 PM Closing Remarks
6:00 PM – 7:00 PM Happy Hour Reception
https://www.eventbrite.com/e/wuxi-apptec-workshop-2019-discovery-ind-and-beyond-tickets-54671208055?aff=erelexpmlt
Crowne Plaza-Foster City Hotel 1221 Chess Dr Foster City, CA 94404
Please join us at the BioPacific Toastmasters club contest of 2019! It will be a guaranteed event of excitement and joy as you will see club members applying their best efforts in the contest. We will be providing FREE breakfast, snacks, and drinks!
Toastmaster Evaluation Contest and Open house
What is it: Contestant evaluator will give an evaluation to the same test speaker
Time: 9:45 am - 11:15 am, Feb 09th, 2019
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Toastmaster Speech Contest
What is it: Contestant will present the prepared speech to the audience
Time: 9:45 am - 11:15 am, Feb 23rd, 2019
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Please join us at the BioPacific Toastmasters club contest of 2019! It will be a guaranteed event of excitement and joy as you will see club members applying their best efforts in the contest. We will be providing FREE breakfast, snacks, and drinks!
Toastmaster Evaluation Contest and Open house
What is it: Contestant evaluator will give an evaluation to the same test speaker
Time: 9:45 am - 11:15 am, Feb 09th, 2019
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Toastmaster Speech Contest
What is it: Contestant will present the prepared speech to the audience
Time: 9:45 am - 11:15 am, Feb 23rd, 2019
Location: Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)

Hanhai Biolabs (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
Covalent Inhibitor Drug Discovery & Development: Biology, Chemistry, PK, Safety and Case Studies
Date/Time: February 7, 2019; 8:34 - 17:00
Venue: Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
Data AI National Summit
Date/Time: Feb 6, 2019 - Feb 8, 2019
Venue: Google, San Francisco Bay Area, Silicon Valley, CA 94089
Organization: American Association for Precision Medicine (AAPM)
Details& Registration:
https://www.eventbrite.com/e/dataai-national-summit-dans2019-tickets-52668062593
Google, San Francisco Bay Area, Silicon Valley, CA 94089
Our website registration system is not stable. If you cannot register over iPhone, please try again on a PC computer with Chrome browser (IE browser not working here). If still cannot register, please email info@cabsweb.org
Happy Chinese New Year
Year of the Pig

2019 will be the year of the Earth Pig. The Pig is a representation of diligence, kindness, and generosity. CABS (Chinese American Biopharmaceutical Society) is planning to organize 2019 Chinese New Year Celebration as a return to thank those who support our organization throughout the years. Please join us on Saturday, February 2, 2019, to kick off the Year of the Pig with exciting programs!
11:30 AM - 1:00 PM: Chinese New Year Lunch, authentic Chinese food from Little Sichuan
1:00 PM - 3:00 PM: CABS Chinese New Year Gala
Program includes:
Folk songs, Beijing opera, Pop songs, Dancing, Talkshow, Fashion show, Conundrums, and much more!
There will be prizes for conundrums and several rounds of lucky draws! So many prizes are waiting for you! Come to meet old friends, make new friends and have fun! Let’s get together to celebrate year of the pig! Child service is available.
Registration Requirement:
On-Line: $10: Adult, 13 and older; $8: Senior; $2: Kid (12 and under)
On-Site: $15: Adult, 13 and older; $8: Senior; $2: Kid (12 and under)
Please mark your calendar and don’t forget to register online to avoid the potential shortage of food. We look forward to seeing you there!
CABS sincerely appreciate sponsors of this event for their generous supports!
Sponsors
Gold
Ranked top 0.1% nationally, Andy provides value real estate service to home owners and buyers in the Peninsula. Andy is Top 10 in US among 50,000 agents at Berkshire Hathaway Home Services in 2018 and has been the No. 1 agent at Berkshire Hathaway CA Realty for 6 consecutive years from 2013 to 2018. In 2018, Andy sold over 50M in Foster City and had a total sales volume of 80M in the Peninsula. Andy specializes in residential and investment purchase and is a certified relocation specialist. Andy also services non-resident individual buyers and corporate clients from China including arranging mortgage and business loans. Andy and his real estate group also service listings in South Bay, East Bay, and San Francisco.
Andy Gan, Ph.D., Realtor
Berkshire Hathaway CA Realty
650-922-3168
Andy@AndyGan.com
www.PhdRealtorBlog.com
Sliver


Recreation Center, 650 Shell Boulevard, Foster City, CA 94404
BioScience Forum Presents
Jill Helms, DDS, Ph.D.
Professor of Surgery
Division of Plastic and Reconstructive Surgery
Department of Surgery
Stanford School of Medicine
Consulting Chief Scientific Officer
Ankasa Regenerative Therapeutics, Inc.
"Powering Tissue Regeneration for an Aging Population"
|
Abstract |
||||
|
Join us from 6-9 pm to network and hear the latest scientific and business advances in the San Francisco Bay Area biotech community |
||||
|
|
||||
|
||||
|
Pre-Registration ends Monday, January 21st, at 9 pm |
The Holiday Inn 275 S Airport Blvd, South San Francisco, California 94080, USA
Seminar Luncheon: Fluorescence Imaging for the da Vinci Robotic Surgery Platform
Date/Time: January 18, 2019; 11:00-14:00
Venue: Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/eventInfo.aspx?eID=616
Crowne Plaza, Foster City, CA
CABS Entrepreneurship Club Innovation Roadshow
Time: 2-4pm Jan 9, 2019 Wednesday
Location: 755 Sansome St., 4th floor, San Francisco (Shanghai Lingang Innovation Center)
Co-organizer: eChinaChem, SVIEF, CTIC
Program:
2:00-2:10pm Opening Remark: Dr. Huijun Zhou, President of CABS Entrepreneurship Club
2:10-2:22pm Dr. William Cao, Founder and CEO, Gracell Biotechnologies
2:22-2:34pm Vincent Keunen, Founder and CEO, Andaman7
2:34-2:46pm Dr. Sheena Liu, Founder and CEO, Medebound
2:46-2:58pm Dr. Nikolay Sergeev, Co-founder and CTO, AlteraBio
2:58-3:10pm Dr. Xiaoxi Wei, CEO, X-Therma Inc.
3:10-3:22pm Dr. Ned Saleh, COO & Co-Founder, Plasmotica, LLC
3:22-3:34pm Dr. Yonglei Shang, VP, Amberstone Biosciences, Inc
3:34-3:46pm Dr. Chris Chen,CEO, Lyratone
3:46-4:00pm Dr. Jia Shidong, CEO, Predicine
755 Sansome St., 4th floor, San Francisco (Shanghai Lingang Innovation Center)
If you register, please attend. There are limited seats at Morrison & Foerster. Each year many people are on the waiting list of CABS Investor Forum.
If something urgent arises, please cancel your registration ASAP online or send an email to info@cabsweb.org.
Registration is free. Registration and photo ID are required to attend the forum. Registration deadline is Jan 3, 2019.
Chinese American BioPharmaceutical Society (CABS) is pleased to announce that 2019 CABS Investor Forum will be held on January 9th of 2019 in conjunction with JP Morgan Healthcare Conference. In 2018, pharmaceutical innovation, medical technology and healthcare continue to be the major areas of licensing and venture capital investment globally. This forum will feature two panel discussions joined by China- and US-based investors, business development executives, and entrepreneurs. The topics will focus on the US-Asia cross-border licensing and investment trends, opportunities and challenges in life sciences.
AGENDA
8:00am - 9:00am Registration and Networking
9:00am - 9:10am Welcome Remarks by Yan Wang, PhD, President, CABS
9:10am -10:10am Panel Discussion
The policy trends, strategies, opportunities and challenges of investment in bio-pharmaceutical industry
Moderator:
Janet Xiao, PhD, JD, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Panelists:
Joseph Benkert, Senior Advisor, National Security, Morrison & Foerster LLPStella Xu, PhD, Managing Director, Quan Capital
Cheryl Cui, PhD, Partner of Nest.Bio Ventures
John Zhu, PhD, Partner, 6 Dimensions Capital
10:10am -10:30am Networking Break
10:30am - 11:30am Panel Discussion
Review and outlook for biopharmaceutical industry regarding investment, M&A and public offering in US and China including Hong Kong
Moderator: Wendy Pan, PhD, Partner, Sidley
Panelists:
Wei Chang, MBA, Managing Director, Z-Park Fund
Jin Wang, PhD, Founding Partner of Manhattan Capital Group, Venture Partner of Puhua Capital
Mark Noguchi, MBA, Independent Advisor and Former Global Head, Roche Partnering
11:30am - 1:00pm Lunch and Networking
Introduction of Panelists
Joseph A. Bankert, Senior Advisor, National Security Group, Morrison & Foerster LLP

Joseph Benkert is a senior advisor in Morrison & Foerster’s National Security practice group. He advises clients on critical national security matters pertaining to the Committee on Foreign Investment in the United States (CFIUS), export controls, and various regulatory and compliance issues. Mr. Benkert previously served as a leading civilian official in the Department of Defense (DoD) under both the Bush and Obama administrations, including as Assistant Secretary of Defense for Global Security Affairs after being nominated by President Bush and confirmed by the Senate. While at the DoD, Mr. Benkert led the department’s involvement in numerous complex matters before CFIUS. Mr. Benkert’s responsibilities also included managing technology security policy, the reform of export control processes, numerous sensitive nonproliferation projects, and a broad range of other defense-related issues. More recently, Mr. Benkert served as a Vice President of a leading global consultancy group, under former Secretary of Defense William Cohen. Mr. Benkert was a career Navy officer with extensive experience both in operational command and in national security policy formulation and implementation.
Janet Xiao, PhD, JD, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP

Janet Xiao, PhD, J.D., is co-chair of the global Life Sciences Group of Morrison & Foerster LLP and specializes in world-wide patent procurement, patent portfolio management, and strategic planning for life sciences companies. Recognized for her deep understanding of the pharmaceutical and biotech industries and knowledge of IP relating to the life sciences arena (Chambers Global), Janet is highly sought after for complex patent strategic and IP due diligence work. She is instrumental on the strategy development for multibillion-dollar patent portfolios for pharmaceutical clients, and works extensively with U.S. and foreign life sciences clients in conducting IP due diligence in various business settings. Dr. Xiao is among the very few IP attorneys who are both equipped with solid skills in global strategic IP management and knowledgeable about IP issues unique to China and Chinese clients, and is frequently called upon to advise clients on IP issues and conduct IP due diligence in U.S./China cross-border transactions and operations in China.
Li Chen, PhD, Founder, Executive Director, Chief Executive Officer, and Chief Scientific Officer, Hua Medicine
Dr. Li Chen, Founder, Executive Director, Chief Executive Officer, and Chief Scientific Officer. leads Hua Medicine a clinical stage biotech company whose mission is to bring personalized diabetes medicines to patients in China and worldwide. He is a pioneer in collaborative innovation in China and advanced Hua Medicine with an operation principle of high standards, high quality and create high value. Hua Medicine leverages global resources to develop GKA and completed 6 clinical studies in China and USA, in which the HMS5552, a novel GK PAM demonstrated desirable safety and efficacy profiles together with an improvement of beta cell function in Chinese T2DM patients. Under his leadership, Hua Medicine completed successfully a POC study and initiated China NDA enabling process. During this period, Hua Medicine raised 200M USD and established the leading position of Hua Medicine in China biotech industry.
Li received his PhD at Iowa State University and joined Roche R&D center in USA in 1992. With 18 years at Roche, Li advanced his career from a medicinal chemist to the head of High Throughput Technology, and later CSO of Roche China R&D Center with a membership at Roche Research Leadership Team. He is an inventor of 35 granted patents and an author with over 60 publications.
Wenseng Pan, PhD, Partner, Sidley Austin LLP
.jpg)
Dr. Wenseng "Wendy" Pan is a leading life sciences lawyer and a partner of Sidley Austin LLP. Her practice focuses on both public and private mergers and acquisitions, PE/VC financings and she is frequently involved in biotech companies’ pre-IPO restructuring and IPOs. She has represented many well-known PE and VC funds, startup companies, growth stage and mature companies and multinational companies in a number of landmark transactions in the life sciences industry. Her deep experience and unparalleled knowledge about the industry has enabled her to provide value-added services to her clients. An active figure and leader in the US/China life sciences community, she has been recognized as a “deal broker” by BioWorld Today, by IFLR1000 as a leading lawyer and in Who’s Who Legal: Life Sciences (Transactional). She is a member of the Board of Directors of The BayHelix Group. Dr. Pan received her JD and PhD from Columbia University.
Cheryl Cui, PhD, Partner of Nest.Bio Ventures

Trained as a biomedical engineer, Cheryl’s interest is to identify promising technologies that address unmet clinical needs and work directly with founders during the venture building process. She was a former Venture Partner at VentureHealth, a Silicon Valley-based healthcare fund, where she coordinated portfolio companies’ growth strategy in the Asia Pacific. Cheryl previously co-founded a mobile health company, which was successfully acquired in 2012 and led to her selection as one of Canada’s Next 36. Cheryl was also selected one of the US Forbes 30 under 30s in 2018. She currently serves as a Board Member of Rootpath and HiFiBio Therapeutics.
Cheryl earned her joint PhD degree in Medical Engineering and Medical Physics from MIT and Harvard Medical School. Her research in building synthetic gene circuits and molecular systems have lead to publications in Science and Nature journals. Cheryl received her B.A.Sc. in Engineering Science and Biomedical Engineer from the University of Toronto.
John Zhu, PhD, Partner, 6 Dimensions Capital
 Dr. John Zhu, Partner of 6Dimensions Capital. Prior to that, he worked for Wuxi Ventures, Greenwoods Asset, Applied Biosystems, etc. Dr. Zhu has over 15 years of experience in health care industry. He has led 20+ private equity investments, including CStone Pharma, Phoenix Healthcare Group, BGI, Gan&Lee, etc. He earned his MBA from University of California at Berkeley and PhD from University of Massachusetts Medical Center.
Dr. John Zhu, Partner of 6Dimensions Capital. Prior to that, he worked for Wuxi Ventures, Greenwoods Asset, Applied Biosystems, etc. Dr. Zhu has over 15 years of experience in health care industry. He has led 20+ private equity investments, including CStone Pharma, Phoenix Healthcare Group, BGI, Gan&Lee, etc. He earned his MBA from University of California at Berkeley and PhD from University of Massachusetts Medical Center.

Dr. Stella Xu is Managing Director of Quan Capital, a life sciences venture fund with offices in China & US, and deep expertise in cross-border value creation and global investments. Prior to Quan, Dr. Xu was a core member of the global management team for Roche’s Immunology, Inflammation & Infectious Diseases areas, and VP/Site Head of Roche Innovation Center Shanghai. From 2012-2017, she led the integrated research & early development site of ~200 scientists, successfully delivered a world-class innovative pipeline from Discovery to global Clinical Development in 3 years, and pioneered novel development strategies in China. Prior to her R&D leadership role, Dr. Xu had extensive global business development and licensing experiences across deal types and disease areas. After establishing the Roche Partnering office in Shanghai in 2008, she managed business development activities in APAC (ex-Japan). She also led global due diligence transaction activities and managed key alliances from research collaboration to marketed products in Roche Partnering US for years. Dr. Xu joined Roche from McKinsey US where she led management consulting engagements in diverse healthcare sectors and functional areas from R&D to commercialization. Earlier in her career, Dr. Xu worked in biotech R&D in oncology & inflammation.
Dr. Xu received her PhD in Immunology from Northwestern University, and B.S. in Biophysics from Peking University.
Dr. Xu currently serves on the Board of ARMO BioSciences, Centrexion Therapeutics and Tempest Therapeutics.
Hai Mi, PhD, Managing Partner, Panacea Venture

Dr. Hai Mi is a Founding and Managing Partner of Panacea Venture. Prior to Panacea Venture, a life science VC focuses on early-stage investment and incubation, Hai joined Kleiner Perkins Caufield & Byers China as a Partner in 2015 and focuses on the firm’s life sciences practice.
Prior to joining KPCB China, Hai was a partner at DT Capital responsible for investment in life science, healthcare and other related fields. Hai was a venture partner of SB China Venture Capital (SBCVC) and his investment transactions include BGI, SyMap Medical and Precise Light. Hai was Senior Director of Strategic Planning and then Vice President of Corporate Communications at WuXi Pharma Tech (NYSE: WX) in Shanghai where he played an important role in NYSE listing, and the acquisition of AppTec in US. He also served on the board of Chemspec International (NYSE: CPC) as an independent board director and the chairman of audit committee, and he participated in the privatization of Chemspec. Earlier Hai was a research scientist at Pfizer in the US.
Hai obtained a PhD in Organic Chemistry from New York University, an MBA from The University of Chicago Booth School of Business, and a B.S. in Material Science from Fudan University in Shanghai.
Cynthia Cai, (蔡小嘉) PhD; MBA; M. Eng., Senior Advisor, US Lead in Healthcare, Northern Light Venture Capital

Dr. Cai is a senior advisor for Northern Light Venture Capital, a leading venture capital firm for Healthcare, TMT, and Advanced Technologies. She is focused on early-stage investments in the US healthcare segment. Dr. Cai had over 20 years of experience in leadership positions with one of the world’s most respected biotech companies. As senior director of marketing in the Mass Spec, Division for Agilent Technologies, she was responsible for global thought leader collaboration and solution development for its billion-dollar MS business. Before that, as business development manager and product marketing manager, Dr. Cai was involved in multiple acquisitions and divestitures and led a $500+ million-dollar flagship product development and its global commercialization.
Dr. Cai earned a B.A. and Master of Engineering from Tsinghua University, received her PhD in Chemistry from the University of Massachusetts and her MBA from The Wharton Business School of the University of Pennsylvania. Dr. Cai served as Delaware governor economic advisor, a member of the Board of the Forum for Executive Women (FEW-DE). Dr. Cai has also been a mentor for Wharton EMBA, Tsinghua University Global MBA and Drexel LeBow School of Business for many years. She is a regular speaker at conferences such as Wharton China Business Forum, for topics related to cross-border commercialization and cross-culture career development.
Jin Wang, PhD, Founding Partner of Manhattan Capital Group, Venture Partner of Puhua Capital
 Dr. Wang is an Angel investor & co-founder of famous Medical Social Network Company (丁香圆www.dxy.cn) In late 90s, he joined Paramount Capital (USA), serviced as an Asia/Great China Regional Director, he also provided value-added service as a venture partner & executive for a portfolio company, PolaRx Biopharma. After that, he became a China Head for business & clinical development of a public company, Cell Therapeutic, Inc In 2002, he return to China, started his own VC firm, MCG Group and invested more than 30 biotech or healthcare related company in China and US. Prior to his VC career, He worked for Roche in Oncology Group and Worcester foundation for Experimental Biology for basic research. He is co-founder of SAPA, very active in servicing biotech community and coaching startups He obtained BS from Zhejiang University and Ph.D.in Biomedicine from WPI in US.
Dr. Wang is an Angel investor & co-founder of famous Medical Social Network Company (丁香圆www.dxy.cn) In late 90s, he joined Paramount Capital (USA), serviced as an Asia/Great China Regional Director, he also provided value-added service as a venture partner & executive for a portfolio company, PolaRx Biopharma. After that, he became a China Head for business & clinical development of a public company, Cell Therapeutic, Inc In 2002, he return to China, started his own VC firm, MCG Group and invested more than 30 biotech or healthcare related company in China and US. Prior to his VC career, He worked for Roche in Oncology Group and Worcester foundation for Experimental Biology for basic research. He is co-founder of SAPA, very active in servicing biotech community and coaching startups He obtained BS from Zhejiang University and Ph.D.in Biomedicine from WPI in US.
Mark Noguchi, MBA, Independent Advisor
 Mark Noguchi is an independent advisor to small and large firms in the biotechnology/pharmaceutical sector. He works with local and global companies addressing corporate strategies and business development.
Mark Noguchi is an independent advisor to small and large firms in the biotechnology/pharmaceutical sector. He works with local and global companies addressing corporate strategies and business development.
In 2018, Mark completed a 33-year career with the Roche Group. Most recently he was Vice President and Global Head, Alliance and Asset Management, and also Global Head, Asia and Emerging Markets Partnering for Roche.
He oversaw the Roche staff of Global Alliance Directors, managing over 150 R&D through commercial-stage partnerships with Pharma companies, biotechnology firms, universities, and other healthcare entities, as well as the alliances with Roche Group members Chugai Pharmaceuticals, Flatiron Health, and Foundation Medicine, Inc. Additionally, he managed the out-partnering of commercial and R&D assets.
Mark led the Roche business development staffs located in Tokyo and Shanghai, establishing partnerships for innovative medicines from Asia countries and emerging markets.
In other past positions at Roche, Mark held management roles in Roche business development, portfolio management, pharmaceutical marketing, regulatory affairs, and information technology. He previously worked for a global consulting company and in academia.
Educated as a zoologist at the University of California, Davis, Mark received his MBA from UCLA’s Graduate School of Management.
Wei Cheng, MBA, Managing Director, Z-Park Fund
 MBA of The Wharton School of the University of Pennsylvania (Finance & Healthcare management); EMBA of PBC School of Finance, Tsinghua University (currently enrolled); M.S. in Chemistry, Rochester Institute of Technology; More than 20 years of experiences in finance and healthcare industries;Partner & MD of Z-Park Fund; Previous Director of IC of China Minsheng Investment Group (CMIG);Previous Director of office of the Board of Directors of China Minsheng Bank (CMB);
MBA of The Wharton School of the University of Pennsylvania (Finance & Healthcare management); EMBA of PBC School of Finance, Tsinghua University (currently enrolled); M.S. in Chemistry, Rochester Institute of Technology; More than 20 years of experiences in finance and healthcare industries;Partner & MD of Z-Park Fund; Previous Director of IC of China Minsheng Investment Group (CMIG);Previous Director of office of the Board of Directors of China Minsheng Bank (CMB);
Mrs. Cheng has served at IBD of Credit Suisse (Asia), and BOC International (China), and led transactions of IPO, equity financing, M&A and debt financing for Chinese companies; served at EXELIXIS (a leading biopharmaceutical company in Silicon Valley, USA) for early oncology drug development.
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105-2482
CABS Entrepreneurship Club Seminar
Speaker: Ruming Catherine Liu, Partner, MagStone Law, LLP
Ruming Catherine Liu, the founding partner of MagStone Law, LLP, is a seasoned corporate attorney, with over a decade of experience representing public and private companies and investment banks in corporate and finance matters. In her private company practice, Ms. Liu advises start-up and emerging growth enterprises from formation through acquisition or initial public offering in matters including venture capital financing, equity financing, operational compliance and technology development (including licensing, distribution and other technology transfer matters). She also represents venture capital firms and angel investors in their investments in start-up companies. Ms. Liu is the lead counsel of ZGC Capital Corporation, AVIC Capital, Sinohydro Corporation, China Oceanwide, Donghai Securities, Danhua Capital, Tsingyuan Ventures, SV Tech Ventures, NetPosa, Great Wall Club, SV Tech Ventures, Novumind, PerceptIn, Muscical.ly, Wexfin and AISense. Ms. Liu is admitted to practice in California and New York, and is the mentor for Stanford Technology Entrepreneurship summer session course.
About CABS Entrepreneurship Club:
The Chinese American Biopharmaceutical Society (CABS) is a non-profit organization for professionals in the biopharmaceutical industry. CABS is headquartered in San Francisco Bay Area, California. CABS is a highly influential association with more than 3000 members and subscribers in the life sciences industry. CABS is the largest and most active Chinese biopharmaceutical association in Northern America. CABS Entrepreneurship Club helps entrepreneurs in the CABS community and foster entrepreneurship, innovative culture, and business opportunities in the life science industry between the U.S. and China.
1633 Bayshore Highway, 2nd floor, Burlingame, CA
Seminar Luncheon: Convertible CAR-T Cells with a Steering Wheel, Accelerator and Brake for Cancer Therapy
Date/Time: December 14, 2018; 11:00-14:00
Venue: Crowne Plaza, Foster City, CA
Organization: PBSS (Pharmaceutical and BioScience Society)
Details& Registration: https://www.pbss.org/aspx/homeSF.aspx
Crowne Plaza, Foster City, CA
BioScience Forum Presents
Tito Serafini, Ph.D.
Chief Strategy Officer and Founder
Atreca
"The Patient Knows Best: Next-Generation Oncology
Immunotherapeutics from Patient Immune Responses"
Abstract
We have recently seen dramatic improvement in patient outcomes from cancer therapies that engage a patient’s own immune response to destroy tumor tissue. Response rates in some indications have risen from single digit percentages to over 50%, with complete responses frequently observed. This may represent just the tip of the iceberg, and novel therapeutics and therapeutic modalities are necessary to continue making progress. A vigorous academic and industry-wide effort is underway in an attempt to turn metastatic cancer into a treatable disease.
Atreca is a privately held company developing novel therapeutics based on a deep understanding of the human immune response. Atreca’s unique discovery platform enables an industrialized identification of functional antibodies generated in the active immune responses of cancer patients responding to therapy. Analyses of such antibodies have led to the identification of over 1,300 patient antibodies that target non-autologous tumor selectively across multiple tumor types. This library of tumor-targeting antibodies is being used to develop therapeutics designed to treat large patient groups. Atreca’s first program to emerge from its platform, ATRC-101, is anticipated to enter clinical development in 2019, and has the potential to treat patients across a wide range of indications via a novel mechanism of action.
Biography
Dr. Serafini is one of the three principal founders of Atreca and was CEO from the Company’s inception in 2010 until 2018. In his role as Chief Strategy Officer, Dr. Serafini is responsible directly for research, preclinical development, technology, and intellectual property. He is a member of Atreca’s Board of Directors.
Before co-founding Atreca, he was Chief Scientific Officer of Nuon Therapeutics, a development-stage biotechnology company. Previously, Dr. Serafini was a founder of Renovis, a public biotechnology company, where he served as an executive officer in multiple technical and business management roles, including leading the research and M&A functions.
Prior to founding Renovis, Dr. Serafini was an award-winning faculty member in the Department of Molecular and Cell Biology at the University of California, Berkeley, where he established the university’s Functional Genomics Laboratory.
Dr. Serafini received a B.S. in biochemistry from Case Western Reserve University and a Ph.D. in biochemistry from Stanford University (advised by Dr. James Rothman), and he performed postdoctoral research at the University of California, San Francisco in the laboratory of Dr. Marc Tessier-Lavigne.
| Join us from 6-9 pm to network and hear the latest scientific and business advances in the San Francisco Bay Area biotech community 6 pm - 7 pm networking 7 pm - 8 pm dinner 8 pm - 9 pm presentation |
||||
| Register Here |
||||
Event Registration ($3 service fee will apply) |
||||
|
The Holiday Inn 275 S Airport Blvd, South San Francisco, California 94080, USA
The advent of many creative and innovative therapeutic platforms such as antibody-drug conjugates (ADC), bispecific proteins as T cell engagers (BiTEs, DARTs, BisAbs, etc), scFv, CAR-T and many others have provided a plethora of new potential therapeutic options for patients. While these new innovative platforms may show great promise for patients they also present unique development challenges to the biopharm industry, especially in the areas of constructing and executing a preclinical safety program that will help to understand and mitigate the safety risks, determination of the starting dose in first-in-human trials of these very potent agents, understanding how target and disease burden play a role in patient selection and safety assessment and ultimately, how all of this is taken into account by Health Authorities around the world.
The objective of this symposium is to showcase the recent success and emerging trends of preclinical and early phase drug development efforts by leading experts from pharma, biotech and academia to encourage discussions and networking opportunities for our CABS members and other biopharma professionals in the Bay Area.
RSVP is required to reserve a seat. NO on-site registration. Lunch will be provided. Please register by Dec 7.
Agenda:
9:30AM - 10:00AM Registration and Check-in
10:00AM -10:10AM Welcome remarks and introduction of CABS (Yan Wang, PhD, CABS President)
10:10AM - 11:10AM Challenges and Approaches in the Early Development of Biologic Therapeutics
Speaker: Hugh M. Davis, PhD, Chief Business Officer (CBO), in charge of Business Development, Sales, Marketing and Strategic Partnerships for Frontage Laboratories, Inc.
11:10AM - 12:10PM Monoclonal Antibody Therapies for Infectious Diseases
Speaker: Steven Chamow, PhD, Vice President of Development at Aridis Pharmaceuticals, Inc.
12:10PM - 1:00PM Lunch
1:00PM - 2:00PM Early Clinical Development in the US - Experiences with Chinese Pharmaceutical Companies
Speaker: Yuanchao (Derek) Zhang, PhD, President, Alavanda Regulatory & Drug Development Consulting, Inc.
2:00PM - 3:00PM Considerations for Early Stage Trials from a Clinical Pharmacology Perspective
Speaker: Mark T. Marino, MD, Chief Medical Officer at Imugene Ltd.
3:00PM - 3:15PM Coffee break
3:15PM - 4:15PM Panel discussion
Moderator:
Cheni Kwok, PhD, CLP, Managing Partner and Founder, Linear Dreams
Panelists:
Hugh M. Davis, PhD
Mark T. Marino, MD
Yuanchao (Derek) Zhang, PhD
Steven Chamow, PhD
Cuiping Chen, PhD
4:15PM - 4:45 PM Networking
Abstracts and Speakers Introduction
1. Presentation Title: Challenges and Approaches in the Early Development of Biologic Therapeutics
Contents:
- The many forms of biologic therapeutic platforms and their development advantages and challenges
- Preclinical safety & toxicology assessment of protein biologics
- How to approach the starting dose for first-in-human studies
- Immunogenicity assessment in regard to biologic efficacy and safety
- Wrapping up all of the preclinical information into an IND package
Speaker: Hugh M. Davis, PhD, Chief Business Officer (CBO), in charge of Business Development, Sales, Marketing and Strategic Partnerships for Frontage Laboratories, Inc.
 Hugh has over 30 years of experience in the pharmaceutical industry. His most recent position was with Johnson & Johnson where he has served as Vice President and Head of Biologics Development Sciences in the Janssen BioTherapeutics (JBIO) division of Janssen R&D, LLC since 2001. JBIO is responsible for creating and characterizing all biologic assets for J&J, across all therapeutic areas. In this role, Hugh participated in the development and approval of many biologic therapies including Remicade®, Stelara®, Simponi®, Sylvant®, Darzalex® and Tremfya®. Prior to J&J, Hugh led the Pharmacodynamics & Exploratory Research Laboratory in the Clinical Pharmacology Unit at Glaxo SmithKline from 1996 to 2001.
Hugh has over 30 years of experience in the pharmaceutical industry. His most recent position was with Johnson & Johnson where he has served as Vice President and Head of Biologics Development Sciences in the Janssen BioTherapeutics (JBIO) division of Janssen R&D, LLC since 2001. JBIO is responsible for creating and characterizing all biologic assets for J&J, across all therapeutic areas. In this role, Hugh participated in the development and approval of many biologic therapies including Remicade®, Stelara®, Simponi®, Sylvant®, Darzalex® and Tremfya®. Prior to J&J, Hugh led the Pharmacodynamics & Exploratory Research Laboratory in the Clinical Pharmacology Unit at Glaxo SmithKline from 1996 to 2001.
Hugh has published over 75 manuscripts in refereed journals, book chapters and invited review articles in areas of therapeutic drug discovery, clinical pharmacology and development in immunology, oncology, metabolic disease, bone metabolism and cardiovascular medicine.
Following receipt of his Bachelor’s degree in Chemistry from Gannon University in 1980 and Master’s and Doctorate degrees in Biochemistry from Villanova University in 1983 and 1985, Hugh completed a Post-Doctoral Fellowship at Centocor, Inc. where he patented the characterization of the CA 125 cancer antigen, a marker used in the diagnosis of ovarian cancer. Hugh has taught Chemistry, Biochemistry and Physics at Delaware County Community College, Immaculata University, Thomas Jefferson University and for 30 years at Villanova University, primarily teaching Allied Health majors in General, Biochemistry and Organic Chemistry. Hugh is a member of the Villanova University College of Liberal Arts and Sciences Dean’s Advisory Council.
2. Presentation Title: Considerations for Early Stage Trials from a Clinical Pharmacology Perspective
Speaker: Mark T. Marino, MD, Chief Medical Officer at Imugene Ltd.

Dr. Mark is drug development professional with experience spanning preclinical research, first in man, experimental medicine, phase 2 and phase 3 studies. His leadership experience includes large international groups as well as being an individual contributor within small biotech organizations. Major achievements include in the following three areas:
Clinical Development: NDAs and sNDAs for Afrezza®, Clarinex®, Banzel®, Acipex®, Aricept® and CL-108. Global Head of Daiichi-Sankyo Mirogabalin Development Team, Worldwide Phase 3 Trials for 3 Indications (6,000+ subjects) in 3 Regulatory Areas (FDA, EMEA, MHW). FDA Advisory Committee meetings for Claritin® and Actemra®.
Clinical Pharmacology: 10+ INDs across multiple therapeutic categories including endocrinology, rare-diseases, neuroscience, oncology, cardiology and transplantation leading teams to POC. Multiple pharmacokinetic, pharmacokinetic - pharmacodynamic and population pharmacokinetic, pharmacokinetic - pharmacodynamic analysis for regualtory submissions and publication. Data analysis with Win-NonLin, NONMEM, R, Python, and SAEMIX.
Mergers and Integration Team Management: Managed Integration Teams between Daiichi-Sankyo and Ambit and Daiichi-Sankyo and Charleston Laboratories, which resulted in strategic collaboration that managed a global development program for CL-108 (novel treatment for Opioid induced nausea and vomiting) and a JAK-2 asset.
3. Presentation Title: Early Clinical Development in the US - Experiences with Chinese Pharmaceutical Companies
Speaker: Yuanchao (Derek) Zhang, PhD, President, Alavanda Regulatory & Drug Development Consulting, Inc.
 Derek has 20 years of combined experience in drug development and regulatory review/approval, including 4 years of industrial experience at Pfizer, 6 years of regulatory review and approval experience at U.S. FDA, and 10 years of experience in drug development and regulatory consulting (4-year executive experience at Frontage Laboratories as Vice President and Head of Drug Development and Regulatory Consulting).
Derek has 20 years of combined experience in drug development and regulatory review/approval, including 4 years of industrial experience at Pfizer, 6 years of regulatory review and approval experience at U.S. FDA, and 10 years of experience in drug development and regulatory consulting (4-year executive experience at Frontage Laboratories as Vice President and Head of Drug Development and Regulatory Consulting).
Derek was Senior Clinical Pharmacology Reviewer in the Office of Clinical Pharmacology, the Center for Drug Evaluation and Research, the Food and Drug Administration (FDA). He reviewed numerous NDAs including several NME NDAs and one BLA, NDA supplements, and IND submissions (both drugs and biologics). He also contributed to several FDA guidance development and revision. Derek lectured at Pharmaceutical Education & Research Institute, Inc. (PERI)’s Premarketing Clinical Drug Safety and Risk Assessment Course many times.
Derek is currently an independent pharmaceutical and regulatory consultant. Derek has in-depth experience in drug development plans/strategies and regulatory review and approval requirements. In the past 10 years, Derek has been providing drug development and regulatory consultation to clients in pharmaceutical industry including Chinese pharmaceutical companies for their new drug development programs including 505(b)(2) and biologics in the United States. The consulting scope covers but not limited to the overall drug development strategies/plans and FDA IND filing preparation, FDA interactions from pre-IND meeting, IND filing, EOP2 meeting and pre-NDA meeting, and clinical development programs after the IND opening.
He has over 30 successful IND filings in the past several years and continues to work with clients in the clinical development phases of those drug candidates. Three of those drug candidates are in Phase 2 drug development; one candidate is ready to enter Phase 3 clinical development; and two 505(b)(2) drug products are at the Pre-NDA filing stage now.
Derek also has in-depth experience in monoclonal antibody development and regulatory review/approval area. At Pfizer (Groton, CT), he was one of the team members on Pfizer's first biologics program, a CTLA4 mAb (CP-675,206 (tremelimumab)) IND project team. He gained firsthand experience in preclinical and clinical pharmacokinetics including immunogenicity testing for mAb from pre-clinical to Phase 1. At the FDA, as a clinical pharmacology reviewer, Derek reviewed several mAb INDs and one complete BLA, motavizumab for the treatment of children with respiratory syncytial virus (RSV) illness from MedImmune.
Derek also has extensive experience in designing bioequivalence (BE) studies for generic drug products including protocol design, study report review and formulating strategies to improve in vivo performance from failed BE studies. He has been consulted by many pharmaceutical companies regarding BE study design for ANDAs. Some of the projects targeted both FDA and WHO approval. He was a consultant for USP/WHO PQM on bioequivalence related issues.
Prior to the FDA, Derek was Senior Research Scientist at Pharmacokinetics, Dynamics & Drug Metabolism, Groton Laboratories, Pfizer Global Research & Development, Pfizer Inc.
Derek received his Ph.D. in 1998 from the University of California, San Francisco (UCSF) under Professor Leslie Z. Benet.
Derek is a member of American Association of Pharmaceutical Scientists (AAPS) and American Society for Clinical Pharmacology and Therapeutics (ASCPT). His major scientific interests include exposure/response, drug interactions, interplay of drug metabolism enzymes and transporters, pharmacogenomics, and effect of renal impairment on enzymes and transporters as well as their contributions to decision makings in drug development.
He has authored or co-authored more than 40 papers, book chapters, posters, and invited presentations in the areas of clinical pharmacology including bioequivalence and risk assessment.
4. Presentation Title: Monoclonal Antibody Therapies for Infectious Diseases
 Speaker: Steven Chamow, PhD, Vice President of Development at Aridis Pharmaceuticals. Inc.
Speaker: Steven Chamow, PhD, Vice President of Development at Aridis Pharmaceuticals. Inc.
Steven Chamow, Ph.D., has 30 years of experience in biopharmaceutical product development including monoclonal antibodies, fusion proteins and nucleic acids. He is currently Vice President of Development at Aridis Pharmaceuticals, Inc., responsible for design and manufacture of the company’s mAb-based therapeutic products. During his career, he has contributed to the development of three marketed products (Avastin, Natrecor, Vectibix). Prior to Aridis, he served as Senior Vice President, CMC, at Intradigm Corporation, a private biopharmaceutical company focused on developing RNAi therapeutics (acquired by Silence Therapeutics).
Dr. Chamow was Vice President, Process Sciences, at Genitope Corporation and at Abgenix, Inc., (acquired by Amgen) where he built the company’s process sciences department and helped to lead the design and construction of Abgenix’ award-winning production facility in Fremont, CA (sold by Amgen to Boehringer-Ingelheim to become its first North American production facility). Before Abgenix, he served as Director of Biopharmaceutical Development at Scios, Inc. (acquired by J&J), and as a scientist and senior scientist in process development at Genentech, Inc. (acquired by Roche).
Dr. Chamow was educated at the University of California (UC Santa Cruz, B.A. in biology; UC Davis, Ph.D. in biochemistry), and completed postdoctoral training at the National Institutes of Health. He is author or co-author of more than 50 scientific publications and patents and co-editor of a 1999 book entitled Antibody Fusion Proteins. Dr. Chamow completed a second book on the same topic (Therapeutic Fc-Fusion Proteins) published by Wiley-Blackwell and released in 2014.
5. Moderator: Cheni Kwok, PhD, CLP, Managing Partner and Founder, Linear Dreams
 Dr. Kwok is a senior biopharmaceutical executive with broad operational expertise who has executed over 150 transactions including M&A, strategic partnerships, licensing, divestitures, spin-offs and project financing. Dr. Kwok is the Managing Partner and Founder of Linear Dreams LLC, a management consultancy for the life sciences industry. The firm’s engagements include business/corporate development, in/out-licensing, corporate strategy, portfolio planning, due diligence, search & evaluation of products and technology, for 38 biopharmaceuticals companies, contract research & non-profit organizations, research institutes and investors in USA, Europe, China, Taiwan and Singapore. Linear Dreams completed many assignments focusing on corporate strategy analyses, portfolio analyses, market intelligence and due diligence on behalf of investors. In addition, the firm helped clients to structure and close multiple collaborations, establish license agreements and supported the inception of a multinational life sciences fund. Dr. Kwok currently serves as the Senior Advisor of Shanghai Henlius Biotech Inc., and Hengenix Biotech Inc., Head of Business Development of Immune-Onc as well as the Chief Business Officer of Fochon Pharma.
Dr. Kwok is a senior biopharmaceutical executive with broad operational expertise who has executed over 150 transactions including M&A, strategic partnerships, licensing, divestitures, spin-offs and project financing. Dr. Kwok is the Managing Partner and Founder of Linear Dreams LLC, a management consultancy for the life sciences industry. The firm’s engagements include business/corporate development, in/out-licensing, corporate strategy, portfolio planning, due diligence, search & evaluation of products and technology, for 38 biopharmaceuticals companies, contract research & non-profit organizations, research institutes and investors in USA, Europe, China, Taiwan and Singapore. Linear Dreams completed many assignments focusing on corporate strategy analyses, portfolio analyses, market intelligence and due diligence on behalf of investors. In addition, the firm helped clients to structure and close multiple collaborations, establish license agreements and supported the inception of a multinational life sciences fund. Dr. Kwok currently serves as the Senior Advisor of Shanghai Henlius Biotech Inc., and Hengenix Biotech Inc., Head of Business Development of Immune-Onc as well as the Chief Business Officer of Fochon Pharma.
As Senior Vice President, Corporate Development at Poniard Pharmaceuticals Inc., Dr. Kwok established corporate and business development, strategic and commercial planning, new product planning, competitive intelligence and forecasting functions. Previously, she was Director of Business Development at Celera Genomics Inc., where she led the business development efforts for Celera's small molecule therapeutics, including the divestiture of the oncology pipeline (including Imbruvica® (ibrutinib)) to Pharmacyclics Inc. (now an AbbVie Company). Dr. Kwok held business development positions of increasing responsibility at Exelixis Inc., where she initiated multiple partnerships and served as the alliance manager for the GlaxoSmithKline PLC (GSK) collaboration. Prior to joining Exelixis Inc., she held various research management, technology assessment and alliance management roles at SmithKline Beecham PLC (now GSK).
Dr. Kwok received a bachelor's degree in biotechnology from the Imperial College of Science, Technology and Medicine, University of London, UK, a Ph.D. in human molecular genetics from the University of Cambridge, UK and has earned the Certified Licensing Professional (CLP) credential awarded by the Licensing Executives Society. Dr. Kwok served as the President of the Chinese-American Biopharmaceutical Society (CABS) in 2016-2017, Vice President of Chinese Bioscience Association in 2015 and Chair of California Chinese Unit Leadership Council of the American Cancer Society (CCU-ACS) in 2015-2016. At present, Dr. Kwok serves on the Board of Directors of CABS, and the Standards, Admissions & Recertification committee of Certified Licensing Professional (CLP).
6. Speaker: Cuiping Chen, PhD, Senior Director, Clinical Pharmacology and Early Development at Jazz Pharmaceuticals
 Cuiping “Tracy” Chen, PhD, is a solution-oriented, innovative, servant leader and team player with >20 years of experience in applying knowledge in pharmacokinetics, pharmacodynamics, clinical pharmacology and drug delivery to the discovery and development of safe and effective remedies.
Cuiping “Tracy” Chen, PhD, is a solution-oriented, innovative, servant leader and team player with >20 years of experience in applying knowledge in pharmacokinetics, pharmacodynamics, clinical pharmacology and drug delivery to the discovery and development of safe and effective remedies.
Currently Tracy is a senior director, clinical pharmacology and early development at Jazz Pharmaceuticals. Her roles at Jazz include leading a cross-function early development team for clinical pharmacology study strategy & execution, supporting clinical development, regulatory submission/interaction, medical education, publication, lifecycle management, and IP strategy, reviewing in-license programs, Identifying and managing CROs and consultants in the areas of DMPK/BA/Clin Pharm.
Prior to Jazz, Tracy had worked in both discovery and development in companies including BMS, Pfizer, JNJ, Celgene and Depomed. She is on the Editorial Advisory Board for Drug Metabolism & Disposition and frequent reviewers for Clinical Pharmacology & Therapeutics. She has near 40 peer-reviewed papers, book chapters and patents in the area of DMPK, drug delivery and clinical pharmacology.
DoubleTree Hotel (835 Airport Blvd, Burlingame, CA 94010)
Speakers: Ved Strivastava (Intarcia Therapeutics), Richard Pittner (Escient Pharmaceuticals), Jessica Hawes (FDA), Paul Feldman (Intarcia Therapeutics), Randy Mrsny (University of Bath), Chetan Lathia (BioXcel Therapeutics), Cheryl Stults (C&M Technical Consulting)
Organizers: Doris Zane, PhD (Intarcia)
Registration fee (USD): Regular: $195; Academic: $125; Students & Unemployed: $35;
Organization: PBSS (Pharmaceutical & BioScience Society)
Details & Registration: https://www.pbss.org/aspx/eventInfo.aspx?eID=591
Crowne Plaza Hotel, Foster City
Speakers: Dawn Dufield (KCAS Bio), Hetal Sarvaiya (Stemcentrx Abbvie), Jessy Fan (Amgen), William Haskins (Catalent Biologics-West), Richard Seipert (Genentech), Yihan Li (Sciex), Suk Hyung (Genentech), Jintang He (Genentech)
Organizers: Keyang Xu and Surinder Kaur (Genentech)
Registration fee: Regular: $195; Academic: $125; For unemployed or students: $30
Registration Site: http://pbss.org/aspx/eventInfo.aspx?eID=595
SF Bay Area: Foster City Crowne Plaza
EPPIC Event:
Title: "Brain-machine Interfaces: From basic science and engineering to clinical trials” by Dr. Krishna V. Shenoy
Register at: http://eppicglobal.org
Date: Nov.15, 2018
Venue: Shepherd Mullin
Address: 379 Lytton Ave, Palo Alto, CA 94301, USA
Networking: 6:30 - 7: 30 pm
Main Presentation and Q&A: 7:30 - 9 pm
Brief Biosketch
Krishna V. Shenoy, PhD, is the Hong Seh and Vivian W. M. Lim Professor of Engineering. He is with the Departments of Electrical Engineering and by courtesy, Bioengineering and Neurobiology at Stanford University. He is also a Howard Hughes Medical Institute Investigator. Prof. Shenoy holds a BS in Electrical and Computer Engineering from UC Irvine (1987-1990), a PhD in Electrical Engineering and Computer Science from MIT (1990-1995), was a postdoctoral fellow in Neurobiology at Caltech (1995-2001), and has been on faculty at Stanford since then (Assistant Prof. 2001-2008, Associate Prof. 2008-2012, Full Prof. 2012-2017, Endowed Chair 2017 to present). Prof. Shenoy directs the Stanford Neural Prosthetic Systems Lab (basic neuroscience and engineering) and co-directs the Stanford Neural Prosthetics Translational Laboratory (clinical trials), which aim to help restore lost motor function to people with paralysis. Honors and awards include a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, a Sloan Fellow, a McKnight Technological Innovations in Neurosciences Award, an NIH Director’s Pioneer Award, the 2010 Stanford University Postdoc Mentoring Award, and he was elected a Fellow of the American Institute for Medical and Biological Engineering (AIMBE) College of Fellows. Prof. Shenoy serves on the Scientific Advisory Boards of The University of Washington's Center for Sensorimotor Neural Engineering (a National Science Foundation Engineering Research Center), CTRL-Labs Inc., MIND-X Inc., Inscopix Inc. and Heal Inc. He is also a consultant for Neuralink Corp.
Shepherd Mullin, 379 Lytton Ave, Palo Alto, CA 94301, USA
Healthcare has traditionally focused on solutions that treat the largest number of patients with similar symptoms. Approaches that do not consider individual differences in genomes, environments, and lifestyles are becoming increasingly obsolete. The “one-size-fits-all” method is not working anymore. Instead, personalized treatments hold the key for the healthcare and pharmaceutical industry of the future.
In 2015, President Obama launched the Precision Medicine Initiative, a bold new research effort to revolutionize healthcare. A range of new technologies have been proposed to achieve this goal, including CRISPR-Cas9, Zinc Finger, and advanced next generation sequencing techniques.
Artificial intelligence will be fundamental to the data-intensive techniques of precision medicine and is producing a paradigm shift to healthcare research and delivery. Machine learning algorithms are being applied to analyze and draw insights from the massive amount of data generated the health industry.
In our Artificial Intelligence and Precision Medicine seminar we have invited a number of biotech companies at the frontier of this movement and will introduce you to the latest developments in this exciting field. Want to catch the wave? Come and Join us!
Schedule
1:00 to 1:30 PM Registration
1:30 to 2:00 PM Precision Genome Engineering for Personalized Mutation-Targeted Adoptive T Cell Therapies for Cancer – A Bold New Frontier from Alex Franzusoff, Ph.D., CEO, Pact Pharma
2:00 to 2:30 PM Augmenting Drug Discovery and Development through Robotics and Artificial Intelligence from Prasun Mishra, Ph.D., CEO and founder, Agility Pharmaceuticals
2:30 to 3:00 PM Creating Effective Drugs through the Use of Big Data and AI from Gini Deshpande, Ph.D., CEO, NuMedii
3:00 to 3:15 PM Break
3:15 to 3:45 PM Time for a Break –Genome Editing Takes the Stage from Thomas Wechsler, Ph.D., Senior Director, Sangamo
3:45 to 4:15 PM Hacking The Genome: CRISPR Gene Editing Has the Potential to Transform Medicine from Rachel Haurwitz, Ph.D., CEO and founder, Caribou Biosciences
4:15 to 4:45 PM Ushering Our World into an Era of Globally Accessible Regenerative Medicine and Immunotherapy from Andre Watson, CEO and founder, Ligandal
4:45 to 5:30 PM panel discussion
5:30 to 6:00 PM networking
Companies and speaker profiles
1. Alex Fransusoff, Ph.D., CEO, Pact Pharma

Dr. Franzusoff joined PACT Pharma in 2017 to develop personalized adoptive T cell therapies using precision genome-engineered patient T cells that target confirmed tumor neoepitopes.
Alex has actively contributed to significant advances in immuno-oncology (I-O). As SVP R&D at Bavarian Nordic, his team’s work on cancer immunotherapy was key to the $1B deal with BMS in 2015. Dr. Franzusoff earlier co-founded GlobeImmune, where he served as VP R&D, developing immunotherapy against cancer & infectious diseases. In Boston, Alex was CEO & Director for AZTherapies, developing Alzheimer’s combination drug therapies for Phase 3 studies. He previously led the venture-backed Permeon Biologics developing intracellular biologics. Alex was tenured faculty at the Univ. of Colorado Medical School until 2004.
Dr. Franzusoff received his PhD in Biochemistry from SUNY Stony Brook, NY. Dr. Franzusoff trained as a postdoctoral fellow with the Nobel Laureate, Dr. Randy Schekman, at UC Berkeley.
2. Prasun Mishra Ph.D., CEO and founder, Agility Pharmaceutical

Dr. Mishra (Ex-Genentech, Ex-NCI, Ex-NIH, Ex-Rutgers) is the Founder and CEO of Agility Pharmaceuticals. He is also founding president and CEO of American Association for Precision Medicine (AAPM). He is best known for discovering the role for noncoding RNA in drug resistance and pharmacogenomics. Moreover, his passion for drug development and precision medicine has led to identification of new drug targets, biomarkers, companion diagnostics, and mechanisms of action/resistance of both new and established drugs, which has facilitated development of rational drug combinations in the clinic. In addition to his professional commitments, Dr. Mishra also gives back to his local biotech community by serving as an advisor to several companies in the San Francisco Bay Area. The companies that he has advised have raised multimillion dollars. Notably, he serves as a mentor and advisor to startup accelerators such as IndieBio, Project Zygote and California Life Sciences Association, to help grow California’s life sciences innovation ecosystem. Moreover, discoveries lead by Dr. Mishra and his teams have contributed to the development of new and viable strategies of prevention, diagnosis and treatment of patients in the clinic.
3. Gini Deshpande Ph.D., CEO, NuMedii

Gini Deshpande, PhD, has leveraged her scientific training and business acumen to turn ground breaking technologies into products that benefit patients. She has built and led global, diverse teams to accomplish important business objectives. She founded NuMedii, a next generation biopharma company that pioneered the use of artificial intelligence and advanced data sciences to rapidly discover new precision therapeutics. As CEO, she raised financing from top tier VC firms, structured critical partnerships with several large pharma companies and is spearheading the company’s transition as it develops its own pipeline. Previously, she helped Affymetrix and other companies with market development strategies for their innovative technologies. She led intrapreneurial efforts at Children's Hospital Boston for the creation of new devices for the tiniest of patients and vaccines for the developing world. Gini has also helped commercialize early stage life sciences technologies in research tools, diagnostics & therapeutics and has closed licensing deals worth several million. Gini received her MS from the University of Pune (India), her PhD in Biological Sciences from Purdue University, and did post-doctoral work at Massachusetts General Hospital. She enjoys being a mentor to women entrepreneurs and scientists and relishes their successes.
4. Thomas Wechsler, Ph.D., Senior Director, Sangamo

Thomas Wechsler, Ph.D., currently serves as Sr. Director of Research and oversees Sangamo’s research programs across the Company’s therapeutic focus areas of inherited metabolic disease and hematology. Since joining Sangamo in 2011, he has held roles of increasing responsibility, including Team Leader, Lysosomal Storage Disorders, and Director of Research, Rare Diseases. Prior to joining Sangamo, Dr. Wechsler worked as a postdoctoral fellow in the Department of Chemical and Systems Biology at the Stanford University School of Medicine. Prior to that, from 2005 to 2010, he worked as a postdoctoral fellow in the Genetic Recombination Laboratory (Dr. Stephen C. West; Cancer Research UK) on homologous recombination and DNA repair. Dr. Wechsler received his Ph.D. in Biology from the Ludwig-Maximilians University in Munich, Germany after conducting his Ph.D. thesis work at the University of California, San Francisco.
5. Rachel Haurwitz, Ph.D., CEO and founder, Caribou Biosciences

Rachel is a co-founder of Caribou Biosciences and has been President and CEO since its inception in 2011. She has a research background in CRISPR-Cas biology, and is also a co-founder of Intellia Therapeutics. In 2014, she was named by Forbes Magazine to the "30 Under 30" list in Science and Healthcare, and in 2016, Fortune Magazine named her to the "40 Under 40" list of the most influential young people in business. In 2018, the Association for Women in Science recognized Rachel with the annual Next Generation Award. Rachel is an inventor on several patents and patent applications covering multiple CRISPR-based technologies, and she has co-authored scientific papers in high impact journals characterizing CRISPR-Cas systems. Rachel earned an A.B. in Biological Sciences from Harvard College, and received a Ph.D. in Molecular and Cell Biology from the University of California, Berkeley.
6. Andre Watson, CEO and founder, Ligandal
1633 Bayshore Highway, 2nd floor, Burlingame, CA
Therapeutic antibody continues to be one of the brightest stars in the drug discovery and has dominated the attention across the spectrum of the pharmaceutical industry. An increasing number of pharmaceutical companies have enhanced their efforts in this competitive field. New technologies are being created and developed, while better antibody therapies and clinical candidates are rising along the horizon as potential treatment for various diseases to save and improve human life.
In this seminar, we have invited five experts in the therapeutic antibody drug discovery research to tell you their expertise and what are the latest advancement of the science and technology that you may consider to apply to develop your own antibody candidates.
Date:Nov. 3rd, Saturday, 1:00-6:00 PM.
Location:Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Agenda:
1:00 to 1:20 PM Check-in and networking
1:20 to 1:30 PM Welcome remarks from CABS.
1:30 to 2:10 PM AID and Phage Display in the Generation and Maturation of Therapeutic Antibodies. Mason Lu, PhD, Founder and CEO, MedAbome, Inc.
Abstract: Provide a brief review on the basic science of antibody molecules and the cutting-edge technologies for the development of therapeutic antibodies. Focus on the application of AID, APOBEC3 deaminases and phage display technology in antibody generation and maturation in the field of biopharmaceutical research and development.
2:10 to 2:50 PM A High Throughput and Versatile Single Cell Antibody Discovery Platform – AbTHENEUM. Chun-Nan Chen, PhD, CEO & CSO, Single Cell Technology, Inc.
Abstract: The best chance to find a low frequency hit is to capture a broad diversity of the immune response. Single Cell Technology uses affinity-maturated B cells on a platform that collects metadata per cell: binding activity against multiple screening molecules is correlated with native VH and VL sequence pairs. Case studies from antibody campaigns will be presented.
2:50 to 3:00 PM Break
3:00 to 3:40 PM Antibody Discovery: From Hybridoma, Phage Display to Microfluidics. Yonglei Shang, MD, MSc, Head of Antibody Therapeutics, Amberstone Biosciences, LLC.
Abstract: Therapeutic antibodies have become an increasing significant component in treating wide range of human diseases. Methods of therapeutic antibody discovery have greatly evolved over time. Hybridoma, phage display and B cell cloning are the main technologies for antibody discovery in the industry. These methods have relatively limited repertoire sampling capacity. Emerging technologies such as deep sequencing and microfluidics/microchamber have been integrated in the antibody discovery process to enhance the sampling of immune repertoires for rapid and high-throughput discovery of optimized antibody leads.
3:40 to 4:20 PM Bispecific Antibodies Engineering and their Potential Applications. Qiang Liu, PhD, Director of Antibody Engineering at Twist Bioscience.
Abstract: Monoclonal antibodies (mAbs) have had tremendous successes in treating variety of human diseases, in particular cancer and inflammatory diseases. mAbs are monospecific, having a single specificity to their targeted antigens. Despite the clinical successes, it is becoming ever more challenging to find effective disease modifying therapeutic targets suitable to naked mAbs. To overcome some of the limitation of monospecific antibodies, and to address diseases’ poly-pharmacology, bispecific antibodies (bsAbs) have been created to target two antigens simultaneously. Dual targeting can be used to bind two antigens on the same cancer cell to simultaneously block two signaling pathways, or improve the selectivity of cancer targeting to generate synergistic therapeutic efficacy. One clear success of bsAbs applications is to create new functionality, like the two approved bispecific antibody drugs: Amgen’s Blinatumomab, a CD19 x CD3 bispecific T cell engager for the treatment of Acute Lymphoblastic Leukemia, and Roche’s HEMLIBRA®which bridges factors IXa and X in the blood clotting cascade for the treatment of hemophilia A.
4:20 to 5:00 PM Single-domain Antibodies from Camelids and Sharks and Their Applications. Naibo Yang, PhD, SVP & COO of GenoImmune Therapeutics Inc (A BGI company).
Abstract: Single domain antibodies can be produced by scFv, but more easily, it can come directly from heavy-chain antibodies. Unlike traditional heavy/light chain paired antibodies found in most mammals, heavy-chain (only) antibodies were discovered in camelids (hcIgG) and sharks (IgNAR). These antibodies consist of only a heavy chain, and are more stable in higher temperature or high salt conditions.
Single-domain antibodies and its derivatives can be used in both diagnostics and therapeutics. The small size and higher stability is very suitable for being used as diagnostic reagents. Using single-domain derived structures can easily modify the avidity and apparent affinity of the antibody. Unique binding domain structures also help to reach hard-to-access active site. In some cases, this can be used to design novel targeted antibody drugs. The easy-to-manipulate structure is also a perfect tool to incorporate into bispecific antibodies or novel CAR-T designs.
5:00 to 5:40 PM Personal Journey to Antibody Drug Development. Haichun Huang, CSO, NovaRock Biotherapeutics.
Abstract: As the co-inventor of Nivolumab (anti-PD1 mAb) and Ofatumumab (anti-CD20 mAb), I will cover my personal story and journey in the discovery and development of therapeutic antibodies.
5:40 to 6:00 PM Refreshment and networking.
Speaker Background:

Mason Lu, PhD, is the founder and CEO of MedAbome, Inc. located in Fremont, California. He has been dedicated in the generation and development of therapeutic monoclonal antibodies (mAbs) for over 30 years. He contributed to many well-known therapeutic mAbs, such as Xolair® (anti-IgE, annual sales revenue was $2.3 Billion in 2016), Ligelizumab (anti-IgE, NDA in 2017), Trogarzo™ (anti-HIV, NDA in 2018), TNX-650 (anti-IL13, Phase III), Lampalizumab (anti-factor D, Phase III), and many other mAbs that are currently in pre-clinical to Phase I clinical trials in Genentech. Dr. Lu’s most recent research interest is focused on (1) Antibody diversity in B cell development; (2) Improvement of technologies for mAb generation, live-cell high-throughput screening (HTS), antibody humanization and maturation; (3) Immunotherapy with universal CAR-T for leukemia and solid tumors.
Prior to MedAbome, Dr. Lu was an assistant professor in MD Anderson Cancer Center, a founder of MabStar, Inc. and a cofounder of MabCom, Inc., a visiting professor in Northeast Forestry University and Chinese National Human Genome Center. He had been working as Research Scientist/Research Manager II in Tanox/Genentech, Inc. for 9 years. He has many publications and multiple issued patents on antibody-related work.
Dr. Lu received his medical degree from Shanghai JiaoTong University School of Medicine, M.Sc. degree from Shanghai Cancer Institute, and PhD degree from MRC-LMB and University of Cambridge. His postdoctoral training in neuroimmunology was at Baylor College of Medicine.

Chun-Nan Chen, PhD, is Chief Executive Officer and Chief Scientific Officer of Single Cell Technology, Inc. (SCT) located in San Jose, CA.
Dr. Chen received his postdoctoral training in Biochemistry Molecular Biology from UCSF, his PhD from Michigan State University, and held senior scientific positions at Celera Genomics, Applied Biosystems, Pharmageneis, SyStemix and DesignRx Pharmaceuticals. Dr. Chen is a registered United States Patent Agent.
Dr. Chen leads invention and development of a proprietary antibody discovery platform called AbTHENEUM at SCT and he is the co-inventor of 11 issued patents surrounding that platform technology.

Yonglei Shang, MD, MSc, started his biotech career at Genentech in 2004. During his tenure at Genentech, he has participated or led 16 therapeutic antibody pipeline programs with focus on development of bispecific antibody (TDB) and targeting multiple trans-membrane proteins such as GPCR, ion channel proteins. He also actively involved in technology innovation including establishment of dual expression system in E. coli and mammalian cells, implementation of baculovirus assay for antibody cyno PK prediction. His expertise in therapeutic antibody development includes antibody discovery, humanization, affinity modification and developability optimization etc. Recently, he has joined Amberstone Biosciences as the Head of Antibody Therapeutics, focusing on a cutting-edge microfluidics technology on deep and functional antibody screening.

Qiang Liu, PhD, currently is Director of Antibody Engineering at Twist Bioscience, is highly experienced in advancing biotherapeutics, monoclonal antibodies, ADCs and protein therapeutics, from early discovery to clinical trials. As a scientific leader with 20 years of diverse biotech industry experiences in antibody discovery, characterization and engineering, he has deep understanding of how to successfully develop antibody and protein therapeutics and broad knowledge ranging from target validation, assay development, lead generation to manufacturing cell line generation. He is an innovative thinker with over 16 issued and filed patents covering broad research areas.
Dr. Liu started his industry career as a scientist at Sangamo, later he moved to Amgen as a Principle Scientist and then joined Takeda California as Director of Antibody Technologies, where he had built a fully functional antibody discovery and development team, and is responsible for in-licensing and in-house development of several key antibody technologies to support therapeutic and diagnostic antibody needs. Before joining Twist, Qiang also served as Vice President and CTO at Immune-Onc Therapeutics, Inc.
Dr. Liu received his PhD in Microbiology and Immunology from Duke University and did his postdoctoral work at Scripps Research Institute.

Naibo Yang, PhD, is currently the SVP & COO of GenoImmune Therapeutics Inc (A BGI company), leading the BGI's therapeutics branch focus on cutting-edge cancer immunotherapies, including NeoAntigen-based T cell therapy, CAR-T, TCR-T and monoclonal and bispecific antibody therapies for immune checkpoint inhibitors etc. Previously, Dr. Yang was the Sr. Director of Immunogenetics, Complete Genomics Inc, in charge of the immunological studies employing the NGS technology, and the development of antibody therapeutics as well as immune repertoire studies and applications.
Dr. Yang had experience and played leading roles in various biotech companies in US and China including Exelixis, Affymax, BioChain Institute. He had more than 20 years of the drug discovery research and management expertise. Dr. Yang was the first president of CABS and current board member. Dr. Yang got his PhD in Physiology from Thomas Jefferson University and received postdoctoral training at Stanford University School of Medicine.

Haichun Huang, Before NovaRock Biotherapeutics, Ms. Haichun Huang was Director of Hybridoma Research at BMS/Mederax Redwood City in California. She is the co-inventor of Nivolumab (anti-PD1 mAb) and Ofatumumab (anti-CD20 mAb). The team she built and led was the first to develop therapeutic antibodies using the human immunoglobulin transgenic mouse platform developed by Medexex, and involved in the discovery of more than 200 target antibodies. Currently, these therapeutic antibodies against tumor immunity, tumors, cardiovascular, fibrotic and autoimmune diseases are found in pipelines of major pharmaceutical companies around the world, such as Amgen, Pfizer, Novo Nordisk, Fibrogen, Genmab, etc. These include 18 antibodies that have entered clinical stages and more than 50 antibodies in preclinical phases. As the co-inventor of 133 antibody research and development patents, Ms. Haichun Huang is one of the world's best experts in the discovery and screening of hybridoma antibodies.
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Dear CABS members and non-members,
It is my great pleasure to invite you to the next long overdue Commercial Strategy Workshop. The mission of these series of workshops is to provide an overview of the business decision making process in the commercializing of new therapeutics and medical devices. These workshops will be valuable to anyone interested in learning more about the business side of life sciences and potential career paths.
This workshop will focus on valuing a pre-commercial bio-pharmaceutical asset. We will be examining the framework and methodologies in which private equity and corporate development teams look at and conduct due diligence on clinical stage assets. Specifically, we will be covering:
- Market opportunity assessment frameworks
- Forecast structure inputs for net revenue
- NPV analysis methodology
This workshop will be useful for anyone who's ever been curious on how acquisition prices for pre-revenue molecules come to be, or more broadly how the buy-side thinks about clinical stage assets. The content will be introductory and very much a "valuation 101"; no prior experience in forecasting or due diligence is needed to attend.
Sincerely yours,
Anthony Hsiao
Business and Career Development Committee
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Dear CABS/CBA members and friends,
You are invited to the 2018 CAN graduation ceremony / career panel discussion co-organized by CABS and CBA. We will invite five panelists and other CAN mentors to share their experiences in different sectors in the life science industry. Additionally, we would like to invite all CAN mentors and mentees to celebrate the end of the 2018 CAN program together. Free snacks and drinks will be served and there will be lots of opportunities to mingle and network!
Date and time: October 27, 2018, 2:00pm-4:00pm.
Location: 1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010
Agenda:
1:30-2pm Check-in and social
2pm-3pm Career panel
3pm-4pm Small group discussion/networking
Confirmed panelists include:
Ruhong Jiang, Ph.D. (Cofounder & CEO, Applied StemCell)
Yan Qi, Ph.D. (Patent Agent, Morrison & Forrester)
Haochu Huang, Ph.D. (Senior Scientist, Genentech)
Mark Meng (Clinical Programmer, Pharmacyclics, An Abbvie Company)
Aihua Fu, Ph.D. (President and CEO, NVIGEN Inc)
Please register at: https://docs.google.com/forms/d/1Tm_FPO_pFJPSArgSedHvYQnVeMjlCu9ymednnZPDxQA/.
Look forward to seeing you!
Best regards,
CAN Co-Directors
Jenen Tan (jingwen.tan@gmail.com)
Thomas Wei (wei1202@stanford.edu)
1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010
Agenda:
1:30PM – 2:00PM Check-in, onsite registration and networking
2:00PM – 5:00PM Comparison of Regulatory System between US and China
Speaker: Dr. Dan Zhang, Executive Chairman of Fountain Medical Co. Ltd.
Presentation Title: Comparison of Regulatory System between US and China
Contents:
The speech is mainly to outline the key changes before and after the reform of the Chinese regulatory system, and to compare the current regimes with the United States. The presentation will present an outlook of the possible changes in China in the future. The focus of the speech is on how the US and Chinese companies should leverage these changes to accelerate and improve the success rate of innovative drug development.
Speaker: Dr. Dan Zhang, Executive Chairman of Fountain Medical Development. Ltd.

Dr. Dan Zhang is the Executive Chairman of Fountain Medical Development (FMD), a clinical CRO with 1700 employees operating in China, Hong Kong, Taiwan, South Korea, Japan, UK, India, Philippines, Armenia & USA.
Before founding FMD in 2007, Dr. Zhang was the Head of Clinical Development and Head of Safety Assessment at Sigma-Tau Research Inc., a leading Italian Pharmaceutical Company. Prior to that,He was the Vice President at the Quintiles Transnational Corp., the world largest clinical CRO, and Chairman of Quintiles Medical Development (Shanghai) Company Ltd.,
Dr. Zhang is a member of grant review committee for National Drug Development Fund of China, and is also a consultant for the China Food and Drug Administration (CFDA, now called CNDA). He is a member of ICH E19 Expert Working Group. He is also chairing the committee of Pharmaceutical R&D, China Pharmaceutical Industry Research and Development Association. Dr. Zhang is also a senior consultant for the Chinese Academy of Medical Sciences and Peking Union Medical College. He was a member of the Overseas Expert Committee on New Drug R&D for the Ministry of Science and Technology of China, and was the secretary-general of the Association of “Thousand Talent” Expert from 2011-2017. Dr. Zhang was the former board of directors for the Sino-American Pharmaceutical Association (SAPA) and was the former president of Chinese Biopharmaceutical Association-USA (CBA). Dr. Zhang is chairing the government relationship committee of Bayhelix - an organization whose members are senior executives from life-science organizations in USA and China.
Dr. Zhang received his pre-med training from Peking University and received M.D. from Peking Union Medical College. He then went to the Harvard School of Public Health and received MPH in health policy and management. Then he went to the Wharton Business School of the University of Pennsylvania, where he obtained his master’s degree in healthcare management in 1998.
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
CBA 2018 Annual Conference
Theme: Building Bridges to Biotech Innovations
Conference Highlight:
- Keynote presentations from Dr. Mien-Chie Hung (VP of MD Anderson Cancer Center), Wei Wang (President, Sinopharm Group) and Dr. Timothy Lu (Associate Professor, MIT)
- New trends of regulatory considerations in both US and China
- Special talk from FDA officer
- Digital Health and Big Data
- Investment and Entrepreneurship
- Expected to attract more than 300 attendees from bioscience and biopharma industries
- Networking opportunities with life science professionals and potential clients
- On-site recruitment (Simcere, 先声药业)
Registration: https://www.eventbrite.com/e/cba-2018-annual-conference-tickets-49057123176
Please use the promo code “cabs5off” to get a $5 off for your ticket.
Conference Ticket Price:
Breakfast, lunch and event reception are included in the ticket.
|
Ticket types |
Early Bird (by 9/29/2018) |
Regular (9/29/2018 – 10/5/2018) |
On-site (10/6/2018) |
|
CBA Member |
$35 |
$55 |
$80 |
|
Non-member |
$55 |
$75 |
$100 |
|
Student (with .edu email) |
$10 |
$10 |
$30 |
Conference Agenda:
|
8:00 – 8:30 am |
Registration, Networking, and Exhibitions |
|
8:30 – 8:35 am |
Welcome and Opening Remarks Mark Chen, Ph.D., CBA president |
|
8:35 – 8:40 am |
Conference Introduction Thomas Wei, Ph.D., CBA Conference Chair |
|
Morning Keynote Presentation (Moderators: Mark Chen and Lin Sun-Hoffman) |
|
|
8:40 – 9:20 am |
Marker-guided targeted therapy, PARP inhibitors, and immune checkpoint therapy Mien-Chie Hung, Ph.D. (Basic Research VP, MD Anderson Cancer Center, The University of Texas) |
|
9:20 – 10:00 am |
Entrepreneurship in China Wei Wang (Sinopharm Group, President) *Interpreter: Tom Zhang |
|
10:00 – 10:15 am |
Networking and Exhibitions |
|
Session I: Drug Regulation (Moderator: Kai Zheng) |
|
|
10:15 – 10:45 am |
Regulatory considerations in the IND submission of antibody and related products Audrey Jia, Ph.D. (Principal Consultant, DataRevive LLC) |
|
10:45 am – 11:15 am |
Regulatory consideration for clinical development in product life cycle management Ardelle Ying, Ph.D. (Program Director, Genentech) |
|
11:15 am – 11:45 am |
Accelerate new drug development: Paradigm shift in the post-ICH regulatory systems in China, and US Dan Zhang, Ph.D. (Chairman and CEO, Fountain Medical Development Ltd) |
|
Special Talk (Introduced by: Huifang Li) |
|
|
11:45 am – 12:15 pm |
Career Development for Life – IQ, EQ, and SQ / Career Opportunities in the FDA George Chang, Ph.D.(Senior Pharmacologist, FDA) |
|
12:15 pm– 12:25pm |
Sponsor Presentation (Eirgenix) |
|
12:25 – 1:15 pm |
Lunch, Networking and Exhibitions |
|
1:15 – 1:25 pm |
CBA Scholarship Award |
|
Afternoon Keynote Presentation (Introduced by: Thomas Wei) |
|
|
1:25 – 2:05pm |
Engineering Biological Computers for Human Health Applications Timothy Lu, Ph.D. (Associate Professor, MIT) |
|
Session II: Digital Health and Big Data (Moderator: Michelle Chen) |
|
|
2:05 – 2:35pm |
Online dermatology transition from dermatologists to artificial intelligence Alexander Börve, MD (Founder & CEO, First Derm) |
|
2:35 – 3:05pm |
Genomic revolution and blockchain Huijun Zhou, Ph.D. (Co-founder, Doctor Chain) |
|
3:05 – 3:35pm |
Using Genomic Features to Make Smart Clinical Decisions: the Power of Machine Learning and RNA-seq Jing Huang, Ph.D. (SVP of Bioinformatics and Data Science, Veracyte Inc.) |
|
3:35 – 3:50pm |
Networking and Exhibitions |
|
3:50 – 4:50pm |
Investment and Entrepreneurship Panel Discussion (Moderator: Lin Sun-Hoffman) Jo Shen, Ph.D. (Vivo Capital) Keting Chu, Ph.D.(Lyfe Venture) Jimmy Zhang, Ph.D. MBA (Lily Asia Venture) Guoliang Yu, Ph.D. (Global CEO of CBT Pharmaceuticals) |
|
4:50 – 4:55 pm |
Closing Remarks Thomas Wei, Ph.D., CBA Conference Chair |
|
5:00 – 7:00 pm |
Evening Reception |
Registration: https://www.eventbrite.com/e/cba-2018-annual-conference-tickets-49057123176
Please use the promo code “cabs5off” to get a $5 off for your ticket.
Crowne Plaza, Foster City, CA
Click here to register:
https://www.eventbrite.com/e/ctic-healthcare-symposium--tickets-49882571113
Discount Registration
GUESTCS45 is the code for CABS.
Please use this promotional code. Select "apply code", the rate will be $45.
499 Illinois Street, San Francisco, CA 94158
|
Crowne Plaza, Burlingame, CA
Discount is available for attendees and compensation is available for speakers and chairs.
Dear Friends from Global Biotech Companies,
1. If you have topics to present related to your expertise, your company, your new invention/innovation and other related areas, please contact us ASAP, and we shall try our best to accommodate your presentation in our programme.
2. We are looking for chairs, organizers and panellists for the following topics:
a. China-US Trade War – how does it affect biotechnology and pharmaceutical industry locally, nationally and globally?
b. How to build Hong Kong and the Greater Bay Area into an Asian Biotechnology Hub?
c. How to build and evaluate your biotechnology company so it can be eventually listed in HKEx and US NASDAQ?
3. On the last day of the event (Sept 24), our programme consists of a whole day on business pitching. If you are a normal registered participant and wish to pitch your company in this event, please let us know. If you are an investor, we would like to invite you to be one of our judges as several awards will be given to the pitching companies.
If you are interested, please register and submit your proposal ASAP to agbs2018@hkbio.org.hk.
For further details about this event please visit: http://www.hkbio.org.hk/agbs2018site/index.php/en/.
Facebook: https://www.facebook.com/hkbio/
Phone: +852 2799 7688
We wish to see you in Hong Kong and the Guangdong-Hong Kong-Macao Greater Bay Area next month.
Regards,
HKBIO
P.S. If you have any problems with the registration fee and local expenses, please let us know so that we may be able to help.
L'hotel Nina et Convention Centre, 8 Yeung Uk Rd, Tsuen Wan, Hong Kong
Zhaoyuan Promotion Reception
Time: Sept 9th, 10AM -1PM
Location: Crowne Plaza, 1221 Chess Dr, Foster City, CA 94404
Comlimentary lunch will be served after the presentations of favorable policy initiatives at Zhaoyuan for entrepreneurs.
City of Zhaoyuan
Located in the northwest Shandong Peninsular, Zhaoyuan covers an area of 1,433 km2 with a population of 570,000. It consists of 14 towns (sub-districts) and one national Economic and Technological Development Zone. Zhaoyuan has been honored with many notable titles, including “Gold Capital in China”, “Vermicelli Capital in China”, “Hometown to Red Fuji Apple”, “China Excellent Tourism City”, “National Top 100 Science & Technology Advanced County-level Cities”, and “National Top 100 County-level Cities” etc.
Zhaoyuan has demonstrated strong economic strength. In 2017, it ranked the 38th on the list of Top 100 National County-level Cities in terms of Economic Basic Competitiveness and 33th of Top 100 National County-level Cities in terms of Comprehensive Strength, respectively. The GDP amounted to RMB 74.01 billion and the local public budget revenue reached RMB 5.75 billion. In recent years, based on the traditional five pillar industries of gold, tire and auto parts, electronics, machinery manufacturing and food processing, Zhaoyuan has cultivated certain strategic emerging industries such as bio-pharmaceutical industry, new materials industry, smart manufacturing, cultural tourism and electronic commerce.
Zhaoyuan's Bio-pharmaceutical Industry
For the development of bio-pharmaceutical industry, Zhaoyuan has planned a Bio-pharmaceutical Industrial Park which covers an area of 1 km2, established Governmental Industry Guidance Fund. Zhaoyuan has attracted biophama companies, such as Bellen Chemistry, which integrates R&D, production and service of pharmaceutical API. The city also launched bio-pharmaceutical innovative cooperation with Beijing Business Incubation Association (BBIA) and Zhongguancun Bio-pharmaceutical Industrial Park through strong leadership, renown experts, strong financial support, and complete supportive policies and services.
We sincerely hope that more and more institutions, platforms and enterprises including members of CABS to give supports and actively take part in the development of Zhaoyuan bio-pharmaceutical industry. On Sept 9th meeting, Zhaoyuan commence department will introduce their policy initiative and other favorable financial supports to the startups.
Crowne Plaza - Foster City
Dear CABS members,
I am so pleased to inform you that we have received a total of 38 applications to join the CABS Executive Council (EC) for this new term ! Thank you so much for your strong support and participation. Together, we can launch new initiatives and events to better serve our members and to strengthen our CABS brand.
Now is the time for you to elect your EC. All CABS members are entitled to vote. You can view the profiles of our applicants here. Please kindly login to our website to cast your vote of support for the new EC. We will elect a total of 24 EC members.
Voting will close on September 7 (Friday) at 9PM PT.
We will introduce you to the new EC and highlight our plans for the new term in late September. Please stay tuned !
Thank you very much for voting and we truly appreciate all your support !
Yan Wang, PhD
CABS President-Elect
Email: yan.wang@cabsweb.org
CABS Executive Council (2018-2019) Election Application is Now Open!
Dear CABS members and friends,
CABS has been proudly serving 3000+ members, sponsors and friends of the life sciences communities in the US, China and Pacific Rim countries for the last 20 years. We organize year-round programs and workshops to showcase the latest advancements in life sciences, foster collaborations and entrepreneurship, facilitate mentor-ship and career development, and create networking opportunities for our members.
We are now calling for candidates to run for our Executive Council (EC) for 2018-2019.
Our EC is the heart and soul of CABS, and orchestrates our activities throughout the year. In September, the new EC will be taking the leadership role in planning and organizing exciting new programs and initiatives both locally and abroad. This is your opportunity to lead and to make significant contributions to our organization. With your participation, we can elevate CABS to the next level!
To participate in our election, please kindly submit your application here and the deadline has been extended to August 27, 2018. Any current Active Member (lifetime members and members who paid their membership dues) is qualified for being elected as an Executive Council member.
We will have a separate announcement regarding the voting process.
Very much look forward to working together and really appreciate your kind support !
Yan Wang, PhD
President-Elect of CABS
(626) 272-6619

Titles of Seminars:
1) ICH in China-a reality check and its impact on drug developmentDr. Dan Zhang, Executive Chairman of Fountain Medical Development. Ltd.
Contents:
a) One year has passed since CFDA became a member of ICH, what is the current status of ICH implementations in China?
b) What about derived new regulatory policies, patent consideration and reimbursement policies
c) What are the impacts of these changes on clinical development planning and regulatory strategy across Pacific Ocean?

Dr. Dan Zhang is the Executive Chairman of Fountain Medical Development Ltd, a clinical CRO with 1700 employees operating in China, Hong Kong, Taiwan, South Korea, Japan, UK, India, Philippines, Armenia and USA.
Dr. Zhang was the Head of Clinical Development and was the Head of Safety Assessment at Sigma-Tau Research Inc-leading Italian Pharmaceutical Company. He was the Vice President at the Quintiles Transnational Corp., the world largest clinical CRO, and Chairman of Quintiles Medical Development (Shanghai) Company Ltd.,
Dr. Zhang is a member of grant review committee for National Drug Development Fund of China, and he is also consultant for the China Food and Drug Administration (CFDA). He is a member of ICH E19 Expert Working Group. He is also chairing the committee of Pharmaceutical R&D, China Pharmaceutical Industry Research and Development Association. Dr. Zhang is also a senior consultant for the Chinese Academy of Medical Sciences and Peking Union Medical College. He was a member of the Overseas Expert Committee on New Drug R&D for the Ministry of Science and Technology of China, and was the secretary-general of the Association of “Thousand Talent” Expert from 2011-2017. Dr. Zhang was the former board of directors for the Sino-American Pharmaceutical Association (SAPA) and was the former president of Chinese Biopharmaceutical Association-USA (CBA). Dr. Zhang is chairing the government relationship committee of BayHelix - an organization hosting senior executives from life science organizations in USA and China.
Dr. Zhang received his pre-med training from Peking University and received M.D. from Peking Union Medical College. He then went to the Harvard School of Public Health and received MPH in health policy and management. Then he went to the Wharton Business School of the University of Pennsylvania, where he obtained his master’s degree in healthcare management in 1998.
After received MPH in health policy and management from Harvard School of Public Health, he went to the Wharton Business School of the University of Pennsylvania, where he obtained his Master’s degree in healthcare management in 1998.
2) Legal consideration if you want to set up a company in China or in the US or both
Chuck Comey, Partner, Morrison & Foerster LLP
(650) 813-5723, ccomey@mofo.com

Chuck Comey is an M&A and corporate finance partner in the firm’s Palo Alto office. Mr. Comey’s practice experience includes working on the ground in China and Japan for 16 years. He advises clients on mergers and acquisitions, venture capital and private equity financings, and joint ventures and strategic alliances in the technology, consumer and life sciences sectors.
Beginning in 1994, Mr. Comey was seconded by the firm to the legal department of a major Japanese trading company, and thereafter was resident for nine years in Morrison & Foerster’s Tokyo office, where he specialized in cross-border mergers and acquisitions and strategic alliances. Before relocating to Silicon Valley in July 2010, Mr. Comey opened and served as managing partner of the firm’s Shanghai office from 2003 – 2010. He speaks and reads Mandarin.
3) Legal consideration for cross-border licensing deals, successful stories of cross-border licensing deals
Scott Carter, PhD, Of Counsel, Morrison & Foerster LLP
(415) 268-7884, scarter@mofo.com

Scott Carter, Ph.D., J.D., focuses his practice on the life sciences industry, representing life sciences companies and investors in complex transactions relating to the development and commercialization of biotechnology, pharmaceutical, diagnostic, and medical-device products. He has extensive experience negotiating corporate partnership, licensing, research and development, clinical trial, manufacturing, and supply agreements. Dr. Carter also advises clients on the intellectual property aspects of mergers and acquisitions.
About Morrison & Foerster:
Morrison & Foerster was founded in San Francisco in 1883. Today, with approximately 1,000 lawyers in 16 offices across the U.S., Asia and Europe, Morrison & Foerster offers clients a comprehensive platform of global legal services, including extensive capabilities in the areas of corporate finance, mergers and acquisitions, tax, regulatory & compliance, litigation, and, intellectual property, among many others. We represent more than 550 life sciences companies, research institutions, and venture capital funds and investment banks that finance them. With more than 180 life sciences lawyers around the world, our fully-integrated team has the depth of practice and global reach that are critical in today’s global life sciences market.
4) Introduction of the Investment Environment of Bio-pharmaceutical Industry in Qidong City
Mr. Kangli Wang, Deputy Mayor of Qidong Municipal Committee

Mr. Kangli Wang is the Standing Committee and Deputy Mayor of Qidong Municipal Committee. From 1997 to 2017 Mr. Wang served as a member of the Party Working Committee of Nantong Economic and Technological Development Zone and deputy director of its management committee. Mr. Kangli Wang received his Bachelor Degree of Arts from the department of foreign languages of Fudan University and Master Degree of Public Administration from University of Maryland.
About Qidong

Qidong is located on the north bank of the Yangtze River estuary, adjacent to the international metropolis Shanghai. The expressway G40, Ningqi Railway, Chongqi Bridge and the planned high-speed Railway along the Yangtze River connect Qidong with Shanghai closely. The advantage in time and space allows Qidong to emerge into the one-hour economic circle of Shanghai. Qidong is well-known as the hometown of electric tools, marine economy, architecture, education, longevity and printmaking. Qidong is in the first batch of coastal areas open to the outside in China. It ranks No.22 among national county-level economies, and 11 among small and medium-sized cities and towns across the country. In 2017, the city's GDP reached 14.467 billion dollars and its per capita GDP reached 15,197 dollars. Its research and development investment totaled 379 million dollars, accounting for 2.6% of the city's GDP, and the number of patent applications reached 7286. In addition, there are 152 high-tech enterprises in the city, accounting for 30% of the above-scale enterprises in Qidong.
Life & Health Science City is located at the north bank of the Yangtze River estuary, covering an area of 100 square kilometers. Mainly focusing on bio-medicine and health industry, it is devoted to develop genetic testing, bio-medicine, precision medicine, medical equipment, health and wellness industries, aiming to build the Yangtze River Delta innovation base of life science and technology, cluster of health Industries and a leisure and livable new city with a planned population of 1 million. By the end of 2017, there were 12 pharmaceutical manufacturers and 13 medical device manufacturers settled in the city. The leading companies include Bayer Pharmaceuticals, Gaitian Pharmaceutical, WuXi PharmaTech, ChemPartner, Xidi Pharmaceutical, Hotmed Sciences, Chengxin Pharmaceutical, Dongyue Pharmaceuticals and other well-known bio-pharmaceutical manufacturing companies both at home and abroad. 12 products produced in Qidong have been identified as national key new products, and 65 products have been identified as provincial high-tech products. In addition, there are 3 provincial-level enterprise academician workstations, 6 provincial engineering technology research centers, and 16 Nantong municipal engineering technology research centers in the biomedical field. In 2017, the output value of above-scale enterprises in new pharmaceutical industry in the city was nearly 2.2 billion dollars.
The Executive Deputy Mayor Mr. Kangli Wang and accompanying delegation of Qidong City, and other colleagues in the bio-pharmaceutical field will attend this event and share their experiences.
We sincerely invite you to register for this event. Limited places, please register as soon as possible! Dinner will be provided. RSVP is required for dinner reservation.
启东位于长江入海口北翼,毗邻国际大都市上海,高速公路G40、宁启铁路、崇启大桥及规划中的北沿江高铁将启东与上海紧密相连。时空的优势将启东纳入大上海一小时都市圈。启东是全国著名的电动工具之乡、海洋经济之乡、建筑之乡、教育之乡、长寿之乡、版画之乡。作为全国首批沿海对外开放地区之一,启东位列全国县域经济22位,全国中小城市创新创业百强县市11位。2017年全市GDP达到144.67亿美元,人均GDP达15197美元,研发投入总额3.79亿美元,专利申请7286项,研发投入占全市国内生产总值的2.6%。另外市内还有高新技术企业152家,占全市规模以上企业总数的30%。
启东生命科技健康城位于长江入海口北翼100平方公里内,围绕生物医药及大健康产业,大力发展基因检测、生物医药、精准医疗、医疗器械、健康养生等产业,建设长三角生命科技创新基地,健康产业集聚中心、滨江休闲宜居新城,规划人口达到100万。截至2017年底,全市现有药品生产企业12家,医疗器械生产企业13家,领军企业有拜耳药业、盖天力药业、药明康德、睿智化学、希迪制药、华拓药业、诚信药业、东岳药业等国内外知名的生物医药制造企业。全市有12个产品被认定为国家重点新产品,65个产品被认定为省高新技术产品。另外生物医药领域还有省级企业院士工作站3家、省级工程技术研究中心6家、南通市级工程技术研究中心16家。2017年全市新医药产业规模以上企业产值近22亿美元。
出席此次活动的主要人员有启东市常务副市长王康力先生及随行代表团及生物制药领域其他同行们。
我们诚挚邀请您注册参加此次活动,名额有限,报名从速! 会后提供晚餐,请尽早注册以便统计晚餐人数,谢谢!
Hanhai BioLabs (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
BioPacific Toastmasters Open House
Contact information: Di Xue (xuedi1104@gmail.com)
Do you find your career development limited by speaking skill? Do you know how to persuade audiences through word choice, rhetorical devices, vocal variety, and body language? Do you want to tell the best stories when pitching how your technology transforms industries? Do you want to grow into a confident communicator and a competent leader?
If your answer to any of the questions above is yes, please join the BioPacific Toastmasters' (https://biopacific.toastmastersclubs.org/) open house on Sat, Aug 25th! BioPacific Toastamsters is a part of the Toastmasters International (https://www.toastmasters.org/), a global non-profit organization dedicated to communication and leadership development. We are proudly sponsored by CABS, and we aim to provide a supportive and positive environment where members have the opportunity to practice and learn. In the open house, you will see what our regular meeting is like and how we support members in improving their public speaking skills. Last but not least, free breakfast will be provided!
Please RSVP here by 11:59PM on Thu, Aug 23rd. Look forward to seeing you!
1633 Old Bayshore Hwy #280, Burlingame, CA 94010
You and your family are cordially invited to our Annual Summer Networking Picnics, hosted by CABS Social Life Committee.
BBQ grilled pork, beef, chicken, hot dog, hamburger, salad, soft drinks, fruits, desserts and a lot more!
All you can eat and drink! Plus music and summer breeze!
With a bonus of unlimited FUN and unexpected opportunity for making new friends and social/career interactions!!!
Lucky draws: $25 gift cards and other secret surprises!!!
Online registration is strongly recommended.
Online:
Active CABS member: $5
Non-member: $10
Senior >65 or 6<Child <15: $5
Child < 6: free
Onsite:
Active CABS member: $10
Non-member: $15
Senior >65 or 6<Child <15: $5
Child <6: free
On-line Registration closes on August 18th, 12:00 PM (noon).
.jpg)
Redwood Picnic Area; 30 Twin Pines Lane, Belmont, CA 94002
The medical device regulatory reform in China is changing the pattern and future of the enterprises and the whole industry at an unprecedented pace.
What benefits will the enterprises gain from the reform? How should the enterprises understand and make use of these regulatory advantages?
RAMED China medical device regulatory forum, with its team of experts having years of regulatory experience and coming from world famous medical device firms, will share the up-to-date CNDA requirements with you.
Agenda:
2:00PM - 2:30PM Check-in, onsite registration and networking
2:30PM - 5:00PM Seminars
Titles of Seminars
1. Organizational development for medical devices administration in China
Speaker: Chang Yongheng, Chief Advisor of RAMED. This presentation will share main changes of responsibilities from CFDA to CNDA, a newly organized SAMR duties.
2. To be a winner by regulatory solution for innovative medical devices in China
Speaker: Ellen Jiang, Founder of RAMED. This topic contents of both how CNDA regulates innovative medical devices and how applicant strategically design solution for application.
3. CNDA requirement update for medical devices pre-market approval
Speaker: Christine Jiang, Co-founder of RAMED. Christine will share the newly published CNDA revised draft on medical devices regulation, and what industry can be benefited from this reform.
4. CNDA acceptance for overseas clinical data
Speakers: Christine Jiang & Ellen Jiang. This presentation helps understanding the applicability of clinical data conducted out of China for CNDA registration purpose, and possible options to avoid duplication in clinical trial.
5. China medical device market characteristics
Speaker: Jane Shen. The following contents will be included in this presentation: 1) The number of clinical organizations, classification and their characteristics as a buyer. 2) Sales model, tendering policy and price as well as internet-based new medical system.
Speakers Introductions
Yongheng Chang

- Yongheng Chang is the executive chairman of the China International medical device regulatory forum (CIMDR) of the CCFDIE of CNDA.
- He was director of product registration division of the department of medical devices of SFDA (State Food and Drug Administration),
- Mr. Chang was deputy counsel of the department of medical devices of SFDA, and deputy director general of CCFDIE of CFDA (China Food and Drug Administration) successively.
Ellen Jiang, Founder of RAMED

- Ellen has twenty-two years of experience in China regulatory affairs and compliance for medical devices.
- She was director of regulatory affairs & compliance for BD China (1996-2010) and Thermo Fisher Scientific (2012-2015).
- She is currently member of the 5th SAC/TC136 (national standardization technical committee for clinical laboratory and in vitro diagnostic test system),
- She is the 1st SAC/TC338/SC1 (national standardization technical committee for measurement control and electrical equipment safety laboratory.
Christine JIANG, Co-founder & Regulatory Affairs Director

- Ms. Jiang has 12years+ RAQA working experience in Danaher Leica Microsystems and Thermo Fisher Scientific.
- She has extended experience in active medical device CNDA registration.
- She had worked closely with oversea manufacturer and China local manufacturer for registration strategies.
- She is familiar with ISO 13485, ISO 9001. ISO 9001 Lead auditor.
Jane Shen, Senior Consultant

- Ms. Shen has 20 years+ hands on and leadership experience in China and other Asian markets in medical devices filed.
- Her achievements include work with R&D in product development and building premium brand images for international suppliers at local level.
- She has established highly productive commercial team and distribution networks.
Please register online. Online registration is free. Onsite registration is $10.
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Agenda:
1:00 – 1:15 Registration
1:15 – 1:45 Dr. Jack Chen (Principal Scientist, Pfizer)
Sweet and sour of immunotherapy: how to leverage success, overcome resistance, and develop combination therapeutics.
1:45 – 2:15 Dr. Changyu Wang (CEO, Huamian Biotechnology)
Cancer Immunotherapy, PD-1 and beyond.
2:15 – 2:45 Dr. Ute Hoch (Executive Director, Nektar Therapeutics)
Harnessing Potent Immune Agonist Pathways for Oncology
Dr. Ute Hoch is the head of clinical pharmacology and pharmacometrics at Nektar Therapeutics. She obtained her PhD at the University of Wuerzburg, Germany, and completed a post-doctoral fellowship at UCSF. Prior to joining Nektar Therapeutics, she has held leadership positions at Sunesis Pharmaceuticals, GeneSoft and Kosan Biosciences. She has 15+ years’ experience developing innovative small and large molecule therapeutics.
2:45 – 3:00 Break
3:00 – 3:30. Dr. Juliana Idoyaga (Assistant Professor, Stanford University)
TBD
3:30 – 4:00. Dr. Hong Wan (CSO, ALX Oncology Inc.)
Targeting CD47 with high affinity SIRPa fusion protein to enhance both innate and adaptive immunity against cancer without hematological toxicity.
4:00 – 5:00 Panel Discussion
This event is co-organized by CABS and CBA.

Stanford University, Graduate Community Center, Havana Room, 750 Escondido Rd., Palo Alto, CA 94305
17Talk 2018 Healthcare Investment Future Pioneers Forum
Date: July 7-8
Location: Shanghai, China
Organizer: 17Talk
Speakers and Lecturers:
- Binhui Ben Ni, Operating Partner and CEO, iBridge Capital
- Guoliang Yu, Executive Chairman, CrownBio
- Amy Tang, Venture Partner, Qiming Venture Partners
- Lynn Yang, Managing Director, Sequoia Capital China
- Will Liu, Partner, Eight Roads Ventures
- Yu Zhang, CEO, Aeon Therapeutics
- Liqun Wang, President, Fosun Kite
- Yufen Zheng, Managing Partner and CEO, Yueyin Venture Capital
- Ying Hong, COO, Genetron Health
And more
Online registration is closing soon. Please click on the link for more information and registration this event
Shanghai, China
2018 BioPacific Conference
Theme: Innovation, Collaboration and Globalization
This conference will showcase:
- Winners of 2018 CABS K. Fong Award in Life Sciences
-
Expected to attract more than 500 attendees of life science professionals from the US, China and other regions
-
Opportunities to connect with your peers and potential clients
-
Cutting-edge science and technology in life sciences including cancer immunotherapy, cell therapy, precision medicine, and medical technology
-
Success stories of entrepreneurs and emerging companies
-
Patent and legal considerations in life sciences
-
Latest biopharmaceutical development policies and regulations
-
Cross-border investment and M&A and in life sciences
-
BioPartnering Forum to facilitate interactions including project introduction and talent recruitment
Registration:
Registration online as early as possible is highly recommended. The registration on June 22 and on-site will be $150.
Breakfast, refreshments, lunch and evening reception are included.
| CABS Member | Early Bird Registration (by 6/7/2018) | Regular Registration (6/8/2018- 06/21/2018) |
| General | $60 | $100 |
| Academic | $40 Please use your institution email (.edu) |
$80 Please use your institution email (.edu) |
| Non-Member* | Early Bird Registration (by 6/7/2018) | Regular Registration (6/8/2018-06/21/2018) |
| General | $90 | $130 |
| Academic | $70 Please use your institution email (.edu) |
$110 Please use your institution email (.edu) |
* Join CABS today ($30 for one year membership) to save on registration fee: http://www.cabsweb.org/members/
Confirmed Speakers and Panelists include:
- Nils Lonberg, PhD, Senior VP, Oncology Discovery Biology, Bristol-Myers Squibb
- Chris Chen, PhD, CEO, WuXi Biologics
- Sylvaine Cases, PhD, Vice President, Oncology Scientific Innovation, Janssen Pharmaceutical Companies of Johnson & Johnson
- Li Zhu, PhD, Chief Strategy Officer, Nanjing Legend Biotech and GenScript Biotechnology
- Deborah Charych, PhD, Executive Director, Nektar Therapeutics
- Dan Zhang, MD, MA, MPH, Executive Chairman, Fountain Medical Development (FMD)
- Guoliang Yu, PhD, Executive Chairman, Crown Bioscience; Venture Partner, OrbiMed Venture
- Yuling Luo, PhD, Founder, Chairman, and CEO, Alamar Biosciences
- Scott Liu, PhD, Co-founder, President and CEO, Shanghai Henlius Biotech, Inc.
- Yiding(Mike)Chen, Founder and CEO, ACROBiosystems
- Janet Xiao, PhD, JD, Partner, Co-chair, Global Life Sciences Group, Morrison & Foerster LLP
- Li Feng, PhD, JD, Partner, Finnegan
- Wilson Lee, MBA, Director, Sino-Singapore (Chengdu) Innovation Park Development
- Daniel Pierce, PhD, Senior Director of Translational Medicine, Celgene
- Kenneth Fong, PhD, Chairman, Kenson Ventures
- Qingcong Lin, PhD, CEO, BioCytogen Boston
- Tao Chen, MBA, Co-founder and CEO of Paragon Genomics
- Xueheng (Shawn) Cheng, PhD, Chief Technology Officer, Viva Biotech Ltd.
- John Ning, MD, PhD, Clinical Consultant in Clinical Science and Product Development Oncology for Genentech
- Jimmy Zhang, PhD, MBA, Venture Partner, Lilly Asia Ventures
- Cheni Kwok, PhD, CLP, Managing Partner & Founder, Linear Dreams LLC
- Steven X. Cui, PhD, JD, Owner, TransPac IP
- Huijun Zhou, PhD, FACMG, Founder & CEO, iDNA
- Grace Xu, MBA, Chairperson, Dendreon; Senior Vice President, Sanpower Group
- Liyu Wu, PhD, Senior Director, US Pharmaceutical Business Development and Project Management, Novogene
- Brooke Rock, PhD, Principal Scientist, Amgen
- Rufus Pichler, JD, Partner, Morrison & Foerster LLP
- Jingrong Li, PhD, VP of CMC/Manufacturing, CStone (Suzhou) Pharmaceuticals
- Rajeev Dadoo, PhD, MBA, Partner, SR One
-
Angela Rusakova, Master of Economics, Partner, Deloitte & Touche
-
Xiao-Jun Ma, PhD, Chief Scientific Officer, Advanced Cell Diagnostics
-
Nelson Lin, PhD, MBA, Director, Global Commercial Development, AbbVie
-
Ruhong Jiang, PhD, Co-founder and CEO, Applied StemCell, Inc.
-
Wenyan (David) Shen, PhD, SVP and Head of Biologics Research and CMC, NGM Biopharmaceuticals
Conference Agenda
|
8:00 AM – 8:45 AM |
Registration |
|
8:45 AM – 8:50 AM |
Welcome Remarks Yan Wang, PhD, President-elect of CABS and 2018 BioPacific Conference Organizing Committee Chair |
|
8:50 AM – 9:00 AM |
State of the Society Alex J. Zhang, PhD, MBA, President of CABS |
|
9:00 AM – 9:30 AM |
Combining Discreet Immune Mechanisms for Cancer Therapy: From Concept to Clinical Evidence Nils Lonberg, PhD, Senior VP, Oncology Discovery Biology, Bristol-Myers Squibb |
|
9:30 AM – 10:00 AM |
In-China-for-Global Biologics: The Exciting Story of WuXi Biologics Chris Chen, PhD, CEO, WuXi Biologics |
|
10:00 AM-10:15 AM |
Coffee Break |
|
10:15 AM-10:55 AM |
CABS K. Fong Award in Life Sciences Presenter: Kenneth Fong, PhD, Chairman, Kenson Ventures Guoliang Yu, PhD, Executive Chairman, Crown Bioscience; Venture Partner, OrbiMed Venture Yuling Luo, PhD, Founder, Chairman, and CEO, Alamar Biosciences |
|
10:55 AM – 11:15 AM |
Fireside Chat: Moderator: Cheni Kwok, PhD, CLP, Managing Partner & Founder, Linear Dreams LLC Yiding (Mike) Chen, Founder and CEO, ACROBiosystems Scott Liu, PhD, Co-founder, President and CEO, Shanghai Henlius Biotech, Inc. |
|
11:15 AM – 11:55 AM |
IP Considerations, Cross-border Licensing and Collaboration This session will cover intellectual property considerations in drug development and licensing deals; the stories of cross-border licensing deals and collaborations in drug and device development. Moderator, Janet Xiao, PhD, JD, Partner, Co-chair, Global Life Sciences Group, Morrison & Foerster LLP Panelist, Li Feng, PhD, JD, Partner, Finnegan Panelist, Wilson Lee, MBA, Director, United States, Sino-Singapore (Chengdu) Innovation Park Development Panelist, Steven X. Cui, PhD, JD, Owner, TransPac IP Panelist, Rufus Pichler, JD, Partner, Morrison & Foerster LLP |
|
12:00 PM – 12:45 PM |
Lunch Break |
|
12:45 PM – 1:00 PM |
Celebrating 20 years of CABS’s Excellence |
|
1:00 PM – 1:30 PM |
J&J Approach to Global Innovation Sylvaine Cases, PhD, Vice President, Oncology Scientific Innovation, Janssen Pharmaceutical Companies of Johnson & Johnson |
|
1:30 PM – 2:00 PM |
Innovations, Tools, and Animal Models in Drug Development This session will cover humanized animal model for immune checkpoint inhibitor evaluation; how to design clinical trial based on animal model; the importance of NGS and liquid biopsy in precision medicine and drug development; and affinity selection mass spectrometry as an innovative platform. Moderator, Ruhong Jiang, PhD, Cofounder and CEO, Applied StemCell, Inc. Panelist, Brooke Rock, PhD, Principal Scientist, Amgen Panelist, Qingcong Lin, PhD, CEO, BioCytogen Boston Panelist, Tao Chen, MBA, Co-founder and CEO of Paragon Genomics Panelist, Xueheng (Shawn) Cheng, PhD, Chief Technology Officer, Viva Biotech Panelist, Liyu Wu, PhD, Senior Director, US Pharmaceutical Business Development and Project Management, Novogene |
|
2:00 PM – 2:30 PM |
A world-class breakthrough in blood cancer treatment done by China biotech company Legend Biotech with CART cell therapy Li Zhu, PhD, Chief Strategy Officer, Nanjing Legend Biotech and GenScript Biotechnology |
|
2:30 PM – 3:00 PM |
Developing Innovative Therapeutics This session will cover the program selection for drug development; clinical trial strategies on immune-oncology drug; and companion diagnostics for a successful clinical trial. Moderator, Wenyan (David) Shen, PhD, SVP and Head of Biologics Research and CMC, NGM Biopharmaceuticals Panelist, Daniel Pierce, PhD, Senior Director of Translational Medicine, Celgene Panelist, John Ning, MD, PhD, Clinical Consultant in Clinical Science and Product Development Oncology for Genentech Panelist, Jingrong Li, PhD, VP of CMC/Manufacturing, CStone (Suzhou) Pharmaceuticals Panelist, Xiao-Jun Ma, PhD, Chief Scientific Officer, Advanced Cell Diagnostics Panelist, Nelson Lin, PhD, MBA, Director, Global Commercial Development, AbbVie |
|
3:00 PM – 3:15 PM |
Coffee Break |
|
3:15 PM – 3:45 PM |
The regulatory affairs after the enforcement of ICH guideline by CFDA and the comparison of regulatory affairs between China and US Dan Zhang, MD, MA, MPH, Executive Chairman, Fountain Medical Development (FMD) |
|
3:45 PM – 4:15PM |
Harnessing potent immune agonist pathways for oncology and beyond Deborah Charych, PhD, Executive Director, Nektar Therapeutics |
|
4:15 PM – 4:50 PM |
Panel Discussion: Investment, Acquisition and IPO in Life Sciences. This session will cover cross-border life science investment, Sanpower’s acquisition of Dendreon for $819.9 million; IPO in Hongkong vs. US. Moderator, Huijun Zhou, PhD, FACMG, Founder & CEO, iDNA Panelist, Jimmy Zhang, PhD, MBA, Venture Partner, Lilly Asia Ventures Panelist, Grace Xu, MBA, Chairperson, Dendreon; Senior Vice President, Sanpower Group Panelist, Angela Rusakova, Master of Economics, Partner, Deloitte & Touche Panelist, Rajeev Dadoo, PhD, MBA, Partner, SR One |
|
5:00 PM |
Conference Adjourned |
|
8:45 AM – 5:00 PM |
Conference (Convene room) |
|
8:45 AM – 7:00 PM |
Partnering (Synergy 1 room) |
|
8:00 AM – 7:00 PM |
Exhibition hall (Inspire room) |
|
5:00 PM – 7:00 PM |
Post-conference Reception Sponsored by Diamond Sponsors (Engage room and courtyard) |
CABS would like to express our sincere gratitude to all our sponsors for supporting us. Please see sponsor logo below.
Sponsorship: fundraising@cabsweb.org
Questions? info@cabsweb.org
-------------------------------------------------------------------------------------------
VIP speaker bio and presentation abstract (more later)
Nils Lonberg, Ph.D.
Senior VP, Oncology Discovery Biology, Bristol-Myers Squibb
Bio: Dr. Lonberg is Senior VP, Oncology Discovery Biology at Bristol-Myers Squibb, where he leads drug discovery efforts for both targeted oncology agents and immuno-oncology agents. Dr. Lonberg began his career in the biotech/pharmaceutical industry leading the research group at GenPharm International that developed genetically engineered strains of mice with germline-configuration human immunoglobulin genes. These transgenic animals have been used to discover over three dozen clinical-stage human sequence antibodies, including ten FDA approved products (golimumab, ustekinumab, ofatumumab, canakinumab, ipilimumab, nivolumab, secukinumab, daratumumab, bezelotoximab, and olaratumab). In 1997, GenPharm was acquired by Medarex, which was in turn acquired by Bristol-Myers Squibb in 2009. Beginning in 1998, Dr. Lonberg’s research group focused on antibody therapies that target and modulate immune attenuating pathways to activate patient immune responses to cancer cells (so-called “checkpoint blockade” therapies). Ipilimumab, which began clinical testing in 2000, was the first ever checkpoint blockade cancer therapy to enter clinical development and to gain regulatory approval. Ipilimumab, which gained regulatory approval in 2011, was also the first drug to ever demonstrate a survival benefit for patients with metastatic melanoma in a randomized clinical trial. A second checkpoint blockade cancer therapeutic from Dr. Lonberg’s group, nivolumab, entered clinical development in 2006 and gained regulatory approval in 2014. Dr. Lonberg received his Ph.D. in Biochemistry & Molecular Biology from Harvard University in 1985, where he studied under Professor Walter Gilbert, and he was a post-doctoral fellow at Memorial Sloan-Kettering Cancer Center. Dr. Lonberg was elected to the National Academy of Engineering in 2015.
Presentation Title: Combining Discreet Immune Mechanisms for Cancer Therapy: From Concept to Clinical Evidence
Abstract: The cancer immunotherapy drugs ipilimumab and nivolumab target the immune checkpoint molecules CTLA-4 and PD-1 respectively. While both targets are mediators of immune attenuation, preclinical, and now clinical, data clearly demonstrate that ipilimumab and nivolumab have very different mechanisms of action. The combination of these discreet mechanisms showed early promise in preclinical assays and models. That data led to clinical testing of the combined use of both drugs in multiple different cancer types. The combination is now FDA approved in melanoma and kidney cancer, with clinical development continuing in a variety of other cancers. The mechanism of action of this now validated therapy will be discussed.
Chris Chen, PhD
CEO, WuXi Biologics
Bio: Dr. Chris Chen is currently Chief Executive Officer at WuXi Biologics, a Hong Kong listed public company with market cap of approx. $10+ billion USD. At WuXi he has built a world-class open-access integrated biologics discovery, development and manufacturing platform enabling full spectrum of companies ranging from 2-employee virtual companies to Top 20 global large pharma. WuXi has transformed the global biotech industry by enabling entrepreneurs to build successful biotech companies with no need for investing in infrastructure for people, labs or manufacturing. Under his leadership, WuXi has assembled one of the largest biologics teams with over 2700 scientists enabling 60+ biologics entering into clinical trials per year. WuXi has also pioneered disposable-bioreactor based state-of-the-art commercial manufacturing facility and built the first 30,000L bioreactor capacity in the world using disposables. The globally leading platform developed by WuXi Biologics is now serving more than 200 companies worldwide.
Dr. Chen obtained his dual bachelor’s degrees of chemical engineering and automation at Tsinghua University, Beijing China and his Ph.D. in chemical engineering at the University of Delaware, US. He then gained valuable experience in biologics development, manufacturing and quality in the US, where his previous assignments include director and manager positions at Lilly and Merck. Dr. Chen later joined Shanghai Celgen Biopharmaceuticals as Chief Operating Officer, successfully developed a high-titer high-quality commercial process for biosimilar etanercept and obtained regulatory approval for the program in China in 2011. He chaired multiple conferences in biochemical engineering and mab development in US and China and is frequently invited to speak at multiple conferences. He is also adjunct professor at Shanghai Jiaotong University. Dr Chen is co-author and co-inventor of 60+ publications and patents.
Presentation Title: In-China-for-Global Biologics: The Exciting Story of WuXi Biologics
Abstract: Biologics are transforming recent healthcare by addressing unmet medical needs, biologics discovery, development and manufacturing, however, requires significant expertise and investment. Prior to WuXi Biologics investment in biologics, China lags behind the global standards significantly. Over the past seven years, WuXi Biologics has successfully built a world-class biologics platform, developing 161 biologics concurrently from 100+ global clients. Recent approval of Taimed’s Trogarzo showcased ultimate success of the WuXi Biologics investment as it is the first company in China to obtain regulatory approval for commercial manufacturing of biologics in China.
Sylvaine Cases, PhD
Vice President, Oncology Scientific Innovation, Janssen Pharmaceutical Companies of Johnson & Johnson
Bio: Sylvaine is currently the VP of Oncology Scientific Innovation for Janssen Research & Development and the J&J California Innovation Center. In this role she manages and develops a portfolio of external opportunities that are aligned with Janssen Oncology strategy. Sylvaine has significant experience in the fields of cancer biology and metabolism, with more than 16 years in research and drug discovery in both pharma, biotech, and academia. Most recently, she served as Senior Director in the Global Business Development Group and Head of External Science & Partnering, US West for Sanofi where she managed the US West Team’s biotech deal flow in alignment with the Sanofi R&D therapeutic areas. Her team also built and managed alliances with academic centers in the US West ecosystem. Previous roles include Head of Pharmacology at Arete Therapeutics and Senior Group Leader at Cytokinetics where she led programs in cancer pharmacology and translational oncology. Prior to her move to biotech, Sylvaine held an academic appointment at the Gladstone Institutes at UCSF where she focused her research on metabolism and cancer with an emphasis on the tumor microenvironment.
Sylvaine has a Masters in Bioengineering, Major in Biochemistry from AgroParisTech (National Institute of Agronomy, Paris, France), and a Ph.D. in Molecular Oncology from Institut Gustave Roussy for Cancer Research and the University of Paris VII, France. She spent her post-doctoral years at the Gladstone Institutes/UCSF and additionally holds a Sanofi Business Master Certificate from the London Business School.
Presentation title: J&J Approach to Global Innovation
Abstract: To continue impacting human health positively, the question is not whether but how to foster and sustain innovation. Staying the course is not an option and the need to innovate has pushed private and public institutions to search and develop partnering models that help scientists, clinicians, and entrepreneurs realize their dreams of creating healthcare solutions to improve peoples’ lives around the world. In such a global ecosystem, Johnson & Johnson has established its innovation arm that comprises a venture group, a network of incubators – JLABS - as well as innovation centers staffed with search, evaluation and deal-making experts in the early stage biomedical space. We will describe Johnson & Johnson Innovation (JJI) approach as an anchor to discuss global innovation and collaborations in biomedical sciences.
Li Zhu, PhD
Chief Strategy Officer, Nanjing Legend Biotech and GenScript Biotechnology
Bio: Dr. Li Zhu, Chief Strategy Officer of Legend Biotech and GenScript, is in charge of corporate business strategy, in-license, strategic M&A, and new business development. Li obtained his bachelor degree in China in 1982 and his PhD degree at Stanford University in molecular biology and immunology in 1989. He worked at Clontech Laboratories in California from 1990 to 2000, successfully pioneered the commercialization of yeast two-hybrid system and a series of other advanced molecular biology techniques. He founded and managed Genetastix, a platform biotech company focusing on creating human antibody library in yeast and screening such library with genetic method. He returned to China in 2006 and worked at two biotech companies in Shanghai Zhangjiang High-tech Park before joining GenScript in 2010. He has been with GenScript since then. He has participated in the negotiation and final completion of Legend-Janssen BCMA – targeting CART therapy for Multiple Myeloma collaboration deal in 2017, a landmark business event between China biotech and US conglomerate pharma companies. Li has a business administration certificate from University of California, Berkeley.
Presentation Title: A world-class breakthrough in blood cancer treatment done by China biotech company Legend Biotech with CART cell therapy
Abstract: At 2017 ASCO conference in Chicago, Legend Biotech reported the result of preliminary clinical trials in multiple myeloma with its unique CART cell therapy technology. This report was heralded as one of the biggest news of 2017 ASCO and the most successful cancer treatment in the history. Since then many biggest pharma companies have been attracted to Nanjing for license and collaboration negotiation. By the end of 2017, we have concluded a deal with Janssen Pharmaceuticals for a 50:50 cost-and-profit-sharing, worldwide collaboration contract (70:30 in great China). The upfront payment from Janssen to Legend was $350M with additional escalating milestone payments. This deal also sets a big milestone in business development between a China biotech and a US conglomerate pharma companies. I will describe how Legend biotech develops from ground zero to a world class innovative cancer-fighting player.
Deborah Charych, PhD
Executive Director, Nektar Therapeutics
Bio: Dr. Deborah Charych has held senior scientific leadership positions in both academia at the Lawrence Berkeley National Laboratory and in biotechnology at Chiron Corporation, FivePrime Therapeutics, and now at Nektar Therapeutics. At Nektar Therapeutics, she leads the strategic development of the Immuno-Oncology pipeline and identified a novel approach to modulate the tumor microenvironment using polymer-molecular engineering. This work led to the development of NKTR-214, a biased agonist of the IL-2 pathway, currently in Phase 2 clinical development. Alternative engineering of the IL-2 pathway led to the clinical development of NKTR-358 for autoimmune disease. NKTR-358 is currently in Phase I and successfully partnered with Eli Lilly. Follow-on cytokine and small molecule immune modulators are slated for the clinic in 2018 (NKTR-255, an IL-15 receptor agonist and NKTR-262, a TLR 7/8 agonist). At FivePrime Therapeutics, Deborah led a team that contributed to the clinical development of FP-1039, a pan-FGF inhibitor biologic for oncology in Phase II clinical trials. As Director of FivePrime’s Process Development group, she also led the downstream process development of several complex fusion biologics for oncology and inflammation, scaling from Research to GMP manufacturing. While at Chiron Corporation she initiated and led a large proteomics effort to guide oncology target discovery. She also developed novel combinatorial libraries of peptoids to develop immune adjuvants and drug delivery agents. At Lawrence Berkeley National Laboratory, she assumed an academic leadership role as Principal Investigator, focusing on new polymeric materials for biosensor applications. Deborah’s formal education is in Chemistry, earning a PhD from University of California at Berkeley and a B.S. in Chemistry from Carnegie-Mellon University, Pittsburgh, PA.
Presentation Title: Harnessing potent immune agonist pathways for oncology and beyond
Abstract: Many potent endogenous proteins that activate important biological pathways are difficult to translate into exogenous therapies because of toxicities, poor pharmacokinetics or sub-optimal pharmacodynamics. We have engineered endogenous proteins and exogenous small molecules using polymer technology to enable viable medicines. NKTR-214 is in Phase 2 clinical trials and a key example of how polymer conjugation can bias the well-known IL2 receptor pathway to favor CD8 T cell tumor infiltration over Tregs. Other examples to be discussed are NKTR-255, an IL15 receptor agonist and NKTR-262, a small molecule conjugate that stimulates toll-like receptor (TLR). Each agent has been conjugated in unique ways to elicit desirable and controlled pharmacological and immunological outcomes.Dan Zhang, MD, MPH
Executive Chairman of Fountain Medical Development Ltd
Bio: Dr. Dan Zhang is the Executive Chairman of Fountain Medical Development Ltd, a clinical CRO with 1700 employees operating in China, Hong Kong, Taiwan, South Korea, Japan, UK, India, Philippines, Armenia & USA.
Dr. Zhang was the Head of Clinical Development and was the Head of Safety Assessment at Sigma-Tau Research Inc-leading Italian Pharmaceutical Company, He was the vice president at the Quintiles Transnational Corp., the world largest clinical CRO, and Chairman of Quintiles Medical Development (Shanghai) Company Ltd.
Dr. Zhang is a member of grant review committee for National Drug Development Fund of China, and is also a consultant for the China Food and Drug Administration (CFDA). He is a member of ICH E19 Expert Working Group. He is also chairing the committee of Pharmaceutical R&D, China Pharmaceutical Industry Research and Development Association. Dr. Zhang is also a senior consultant for the Chinese Academy of Medical Sciences and Peking Union Medical College. He was a member of the Overseas Expert Committee on New Drug R&D for the Ministry of Science and Technology of China, and was the secretary-general of the Association of “Thousand Talent” Expert from 2011-2017. Dr. Zhang was the former board of directors for the Sino-American Pharmaceutical Association (SAPA) and was the former president of Chinese Biopharmaceutical Association-USA (CBA). Dr. Zhang is chairing the government relationship committee of Bayhelix-an organization hosting senior executives from life-science organizations in USA and China.
Dr. Zhang received his pre-med training from Peking University and received M.D. from Peking Union Medical College. He then went to the Harvard School of Public Health and received MPH in health policy and management. Then he went to the Wharton Business School of the University of Pennsylvania, where he obtained his master’s degree in healthcare management in 1998.
Presentation Title: The regulatory affairs after the enforcement of ICH guideline by CFDA and the comparison of regulatory affairs between China and USAbstract: CFDA has experienced dramatic changes in last three years and it also became a member of ICH. These changes have profound impact on the global drug development for both domestic and global pharmaceutical companies. including their regulatory and clinical development strategies. This presentation would highlight major regulatory changes, compare & contrast with US regulatory system, and analyze the impact of consolidated changes up to this year. Special attention is paid to the current status of these changes and case studies.
CABS would like to express our sincere gratitude to all our sponsors for supporting us.
Sponsorship: fundraising@cabsweb.org
.jpg)
San Mateo Marriott, 1770 South Amphlett Blvd, San Mateo, CA 94402
|
||||||||||||||||||||||||||||||||||||||||||||||||||
Hilton Shanghai Hongqiao No. 1116 Hong Song East Rd, Shanghai, 201103, China
Announcing 2018 CABS K. Fong Award in Life Sciences
Chinese American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that Dr. Guoliang Yu and Dr. Yuling Luo are the winners of CABS K. Fong Award in Life Sciences for their extraordinary achievements in research, entrepreneurship, and innovation. We will honor Dr. Yu and Dr. Luo in the award ceremony at 2018 BioPacific Conference (20th Annual Meeting of CABS) on June 23, 2017 at the San Mateo Marriott San Francisco Airport located at 1770 South Amplett Blvd., San Mateo, CA 94402.
Dr. Guoliang Yu
Dr. Yu is currently the Executive Chairman of Crown Bioscience and a venture partner at OrbiMed Venture. He has also co-founded several biotech startups, including Epitomics, where he was the Chairman, President, and CEO for 10 years prior to its acquisition by Abcam.
Dr. Yu is an innovator, successful serial entrepreneur, and mentor to biotech founders. In 2009, he was nominated as one of the most influential returned talents by the American Business Weekly. In 2010, he was further selected for the Chinese National Thousand Talent Plan and featured by the Scientist Magazine. In 2011, Dr. Yu was invited to attend the 4th session of the Fourteenth National Committee of the Chinese People’s Political Consultative Conference.
Dr. Yu completed his Ph.D. at UC Berkeley with Dr. Greider, who went on to win the Nobel Prize for their discovery in 2009. He later finished his post-doctoral study at Harvard University. In 1993, Dr. Yu joined Human Genome Sciences and led the discovery of the lupus drug Benlysta that was approved by FDA in 2010. In 1998, Dr. Yu was invited to serve as the Senior Vice President of R&D at Mendel Biotechnology. His scientific contribution was translated into 43 peer-reviewed articles, referenced over 6000 times, and was awarded more than 429 patents.
Dr. Yu is also a life science community leader. He is the founding president of the Chinese Biopharmaceutical Association (CBA) and sits on the boards of several professional organizations in the United States and China, including BayHelix, Chinese-American Bio/Pharmaceutical Society (CABS), National Foundation of Cancer Research, and Ray Wu Memorial Foundation.
Dr. Yuling Luo
Dr. Luo is currently the Founder, Chairman and CEO at Alamar Biosciences, Inc., a new start-up. He is a seasoned executive and successful serial entrepreneur in the life science business. Dr. Luo is the inventor of RNAscope, a breakthrough molecular detection technology that has since become a widely adopted platform for life science research and molecular diagnostics. As Founder, President and CEO of Advanced Cell Diagnostics(ACD), he led the company from concept to successful product launch and commercialization. Under his leadership, ACD has become one of the fastest growing life science business and the company was eventually acquired by BioTechne for $325M in 2016. The acquisition was at 13 times sales revenue, which is probably the highest multiple in the life-sciences industry. Dr. Luo and his team have created a total of $300M value for ACD shareholders. Before ACD, Dr. Luo was a co-founder of Panomics (previously named Genospectra before its acquisition of Panomics), a life science company that was acquired by Affymetrix in 2008. At Panomics, he served various roles including Vice President, Functional Genomics and Chief Scientific Officer and he led the development and launch of all of its flagship products. Prior to Panomics, Dr. Luo was one of the earliest senior scientists at Exelixis, now a $6 billion NASDQ company, where he led a team to identify and validate novel therapeutic targets.
Dr. Luo is a member of NCI’s Ad Hoc Working Group on SBIR charged with providing guidance to increase small business and private sector participation in developing and commercializing novel technologies and products. He is also a member of the peer-review panel for NCI’s IMAT (Innovative Molecular Analysis Tool) program. Dr. Luo has published numerous research papers in top peer-reviewed journals. His inventions are the subject of over 30 issued patents. He received his doctorate in biochemistry from Case Western Reserve University followed by post-doctoral research at the University of Pennsylvania. He obtained his B.S. from the University of Science & Technology, China.
About CABS K. Fong Award in Life Sciences
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria will be based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or the research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the CABS community and to promoting international collaboration in life sciences.
About Dr. Kenneth Fong
Dr. Kenneth Fong has spent the last 32 years in the biotech industry after completing his academic pursuit in biomedical research.
He is best known for founding the biotech company, Clontech in 1984 where he had cultivated it to be one of the largest biomedical tool companies founded by an Asian American in the US (400 people with 65 PhD scientists). Clontech was sold to Becton Dickinson in 1999 and Ken has continued his career as a Venture capitalist with Kenson Ventures that he founded. He has since cultivated more than 10 highly successful entrepreneurs, advising them and working with them on the growth of their companies.
Currently, he sits on the board of 4 biotech companies and he was intimately involved with the M/A and IPO of more than 10 companies that are worth north of $3 billion. These companies range from research tools, medical diagnostics and drug development. In almost all cases, Dr. Fong has been instrumental in providing strategies for sustainable growth, value creation and liquidity. Those successful entrepreneurs have moved on to assume leadership in other start-up and mid-sized companies, which in turn leads to a new generation of entrepreneurs.
Ken has held a number of leadership positions over the years. He served as the President of the Society of Chinese Bioscientists in North America (2006-07) and President of the Bay Area Asian American Manufacturers' Association (AAMA, 1987). He was also the member of the Board of Trustees of the California State University System (2006-13). His philanthropic interests include scholarships to San Francisco State University, the Kenneth Fong-Hearst endowed scholarships to the CSU system and 40 student scholarships to Peking University. In 2006, he was involved with establishing the Fong Optometry and Medical library at UC Berkeley, and more recently an endowed professorship at Stanford University and a technology translation endowed fund at San Francisco State University.
Ken obtained his PhD from Indiana University and his BS from San Francisco State University.
Past recipients of CABS K. Fong Awards
2017: Dr. Yinxiang Wang, Co-founder and CSO of Beta Pharma, for his role in leading development and commercialization of Conmana®, the first small molecule oncology drug specifically targeting cancer cells that is completely developed in China, and Dr. Edgar Engleman, for his pioneering research that was the basis of the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA.
2016: Dr. Gerald Chan, co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, of Stanford University, for his pioneering research in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life- saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth, Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.
online
CABS members 20% discount on regular registration fee. Please use CABS20 as the promo code
|
|
|
|
|
|
|
|
|
|
|

Suzhou, China
2018 Stanford Drug Discovery Conference
April 23-24, 2018
Li Ka Shing Conference Center
Now accepting applications for translational stage projects to be presented to a panel of leading scientists, CEOs, and entrepreneurs.
This is a great way to showcase your company, receive informal feedback from industry experts, and network with industry/academia/VC leaders. There is no financial obligation or guarantee of investment. There will be a non-monetary prize for best product/business plan.
The Shark Tank panel includes Roy Vagelos, MD; George Scangos, PhD; Ram Shriram; Robert Robbins, MD; and Amy Chang
Day 1 | April 23, 2018
Welcoming Remarks and Introduction
Joseph Wu, MD, PhD | Director, Stanford Cardiovascular Institute
Lloyd Minor, MD | Dean, Stanford School of Medicine
David Entwistle | CEO, Stanford Health Care
Dennis Lund, MD | Chief Medical Officer, Lucille Packard Children’s Hospital Stanford
Opening Keynote
Brian Kobilka, MD | "Structure-based Approaches for G Protein Coupled Receptor Drug Discovery"
Q & A Session
Academic Drug Discovery Process
Session Chairs and Moderators: Janet Woodcock, MD, and Joseph Wu, MD, PhD
Kathleen Giacomini, PhD
Kevan Shokat, PhD | "Chemical Tricks for Drugging the Undruggable"
Hugh Rosen, MD, PhD | "Intelligent Intervention: Ozanimod from Hit to NCE at Scripps"
Edgar Engleman, MD | "Treating Disease by Manipulating the Immune System"
Panel Discussion
View From the Top
Ken Frazier | "Inventing for Life: the CEO Perspective"
Joe Jimenez | "Outcomes Based Pricing"
Patrick Soon-Shiong, MD | "Patient as the Biological Factory of the Future"
Bob Bradway | "Moving from 'Break/Fix' to 'Predict/Prevent'"
Brent Saunders | "Using the Innovation Ecosystem to Build a Robust Pipeline"
Sanford Weill | "Philanthropy's Vital Role in Partnering with Healthcare"
Panel Discussion
Fireside Chat
Marc Tessier-Lavigne, FRS, FRSC, FMedSci
Lloyd Minor, MD
Q & A Session
Presentation of Lifetime Achievement Award to Roy Vagelos, MD
Introduction: Paul Berg, PhD, and Ken Frazier
Awardee Lecture: Roy Vagelos, MD | "A Career in Drug Discovery"
Day 2 | April 24, 2018
Keynote Address
Kuldev Singh, MD | Introduction
Janet Woodcock, MD | "Changes on the Horizon for the New Drug Regulatory Program"
Q & A Session
Publishing Drug Discovery
Session Chair and Moderator: Sanjay Malhotra, PhD
Howard Bauchner, MD | "Evolving Issues in Medical Journalism"
John Keaney, MD | "Editorial Considerations in the Evaluation of New Therapies"
Paula Kiberstis, PhD | "Drug Discovery and Science: What's behind the Curtain?"
Joao Monteiro, MD, PhD | "Transparency and Reproducibility in Science: How Journals Can Help"
Round Table Discussion
Funding Agency Perspectives
Session Chair and Moderator: Joseph Wu, MD, PhD
Gary Gibbons, MD | "Charting Our Future Together: Advancing Discovery Science for Public Health Impact"
Maria Millan, MD
Q & A Session
Starting Up
George Scangos, PhD | "Transitioning from Big Pharma to Startup"
Shark Tank-style Panel featuring:
Roy Vagelos, MD
George Scangos, PhD
Ram Shriram
Robert Robbins, MD
Amy Chang
Wende Hutton
Closing Remarks / Poster Prize Award Announcement
Li Ka Shing Conference Center

Sanpower and Dendreon: the New Beginning
Date and Time
Sun, April 22, 2018
2:00 PM – 5:00 PM PDT
Location
Stanford University
1265 Welch Road, 3rd Floor
Palo Alto, CA 94305
Seats are limited, Registration is free but encouraged
The event is organized by CABS
In 2017, Sanpower Group, one of the largest private conglomerates in China, made the biggest spash at JP Morgan Healthcare Investors Conference, announcing the completion of Dendreon from Valeant for $820 million. Dendreon's core product, PROVENGE, is the first and only FDA-approved cellular immunotherapy for prostate cancer. Sanpower plans to promote PROVENGE's penetration outside of the US, starting with China and Southeast Asia.
In recent years Sanpower Group has determined the direction for Healthcare sector's growth, including health care, home care, genetic testing, precision medical and other fields. In fact, Sanpower Group previously acquired AnKangTong, Natali, and A.S. Nursing Company. In 2016, the group announced the acquisition of China Cord Blood Corporation and Shandong Cord Blood Bank, this means that it has emerged as the world's largest cord blood banking operator. The group also owns many medical and health institutions.
Agenda:
Time: 2 - 5 PM, April 22, 2018
2:00 - 2:05 PM: Introduction of CABS, Qiang Gan, Co-Chair of ICC, CABS
2:05 - 2:30 PM: Introduction of Sanpower Healthcare, Philip Bu, Senior VP, Sanpower Group
2:30 - 3:00 PM: Dendreon - Deal Sharing and Future Perspectives, Grace Xu, Chairperson, Dendreon; Senior Vice President, Sanpower Group
Speaker Biographies

Philip Bu
Mr. Phillip Bu is a Senior Vice President of Sanpower Group, concurrently serving as the Board Director of Cenbest(SH,600682),Chairman of the Biomedicine Industry Group and the Health & Senior Care Industry Group. Among other domestic and overseas companies, of which Mr. Bu is in charge, he overseas business, in relation to genetic testing, cell immunotherapy, hospital management and senior care, at Dendreon, Natali, Ankang Tong and More Health, Mecox Lane, Findgene as well as Xuzhou Third People's Hospital. Mr. Bu led many mergers and acquisitions to successfully conclusions at home and abroad. Further, he has spearheaded capital withdrawals from the Chinese securities market.

Grace Xu
As Dendreon Chairman, and Sanpower Group’s Senior Vice President, Ms XU is responsible for setting corporate strategy for the Healthcare sector, and prioritizing future M&A focus. Ms. Xu has 25 years of experience in the Pharmaceutical and Biotech industry, has served a number of global leading companies, Novartis, AstraZeneca, and BMS. She has capabilities and experiences in general management, corporate strategy planning, business development, M&A, and new product planning. She also has extensive international experience, in US, Europe, Middle East and Asia. Ms Xu has a MBA from Rotterdam school of management and a MS from University of Connecticut.

Han Ying, PhD
Dr. Ying has over 25 years pharmaceutical experience in oncology drug R&D, regulatory submission, clinical studies, and in starting and building biotech start-ups. He received his PhD at Stanford University in Cancer Biology Program, completed his post-doctoral training with Drs. Nicholas Restifo and Steven Rosenberg at the US National Cancer Institute. He became a principal investigator at Maxine Dunitz Neurosurgical Institute (affiliated with UCLA), where he oversaw a clinical laboratory conducting Dendritic Cell Vaccine trials for malignant brain tumor. He joined Berlex Biosciences (R&D arm of Schering A.G.) as a project leader in cancer research department, then joined Monogram Biosciences, a personalized medicine company that developed biomarkers for selection of patients for novel targeted drugs. In 2010, Dr. Ying co-founded Immunnova, a biotech company focused on dendritic cell vaccines and antigen-specific T cells. He was awarded grand champion in a national entrepreneurs contest in China. He has consulted for several early or late-stage biotech companies in the field of cancer immunotherapy, including HRYZ (Dendritic cell vaccines) and Sanpower Group/Dendreon. He was the key technical expert in an international M&A team that completed the acquisition of Dendreon by Sanpower group (0.9 billion dollars), acquisition of China Life Science Hong Kong facility. He received Advanced Certificate in Regulatory Affairs USFDA in 2007.
Stanford University 1265 Welch Road, 3rd Floor Palo Alto, CA 94305
Taihu (Mashan) Life science and Healthcare Forum – 2018
Theme: Biopharmaceutical and Financial Innovation
The biopharmaceutical industry is one of the most dynamic emerging industries in the world and the future field of global competition. In order to further promote the reform and development of biopharmaceutical in China and encourage international exchanges and cooperation to discuss Chinese biopharmaceutical industry development plan, the "Taihu (Mashan) Life science and Healthcare Forum - 2018 Biopharmaceutical and Financial Innovation" will be held in Wuxi in April 20-22, 2018.
The theme of the conference is "Biopharmaceutical Innovation and Capital Financing". We will invite top experts, scholars, and industry professionals from global biopharmaceutical research and development innovation and financial fields to discuss clinical oncology, protein antibody research, capital financing for biopharmaceutical innovation, new drug delivery systems, research and evaluation of new vaccines topics, etc.
- Ø Conference Agendas
The two-day conference includes key forum and several tracks and workshops. We will invite domestic and global speakers and panelists from the universities, scientific research institutes, R&D companies, pharmaceutical enterprises, and hospitals and clinics to gather and discuss China life science and healthcare market.
“Taihu (Mashan) Life Science and Healthcare Forum” will have seven tracks for Clinical Oncology, Protein & Antibody, Novel Drug Delivery, Financial and M&A, New Vaccine, and ICH Regulations.
Meeting Arrangement:
|
April 20 |
|
|
April 21 |
Opening Ceremony |
|
Key note speeches from academic, government and industry experts: Antitumor drugs, biomedical innovation, drug targets for clinical treatment, antitumor gene immunotherapy, cellular immunity and treatment, regenerative medicine and tissue Engineering, etc. |
|
|
Biopharmaceutical and Life Science Salon |
|
|
April 22 |
Forum 1: Protein & Antibody Biopharmaceutical Research 360 Forum |
|
Forum 2: Capital & biomedical research and development innovation 360 Forum |
|
|
Forum 3: Clinical Oncology Frontier 360 Forum |
|
|
Forum 4: Novel Drug Delivery 360 Forum |
|
|
Forum 5: Development and Evaluation of New Vaccines |
|
|
Forum 6: ICH Regulation |
|
|
Forum 7:Regenerative Medicine and Innovative Medical Devices |
- Ø Conference website
- Ø Online Registration for CABS
- (Early Bird (before 4/10) discount price for CABS members is $198.4, or 20% discount off regular price of $248. The Standard (after 4/10) discount price for CABS members is $230.4, a discount of 20% off regular price of $288.)
- Ø Contact
- Sammi Wang
- MP: +86-189-6499-9689
- We Chat: 135-1532-0786
- Email: sammi@echinachem.com
Zen Town Convention Center, Flower Sermon Bay, Wuxi, China
Registration is free and encouraged
Shanghai Fosun Pharmaceutical (Group) Co, Ltd and Xuzhou Economic and Technological Development Zone will bring you this exciting networking opportunity with figured with opportunities for collaboration, investment and career development!
The event is organized by CABS
Title Sponsor:
![]()
Shanghai Fosun Pharmaceutical (Group) Co. Ltd. 上海复星医药集团
Founded at 1994, Shanghai Fosun Pharmaceutical (Group) Co. Ltd is becoming a leading healthcare group in China. Its business covers the key sectors of healthcare value chain, including drug manufacture and R&D, medical service, medical device and diagnosis, pharmaceutical distribution and retail. Fosun Pharma has a national enterprise technology center, and has built a high-efficiency chemical innovation drug platform, biopharmaceutical platform, high-value added generic drug platform and cellular immunity platform. So far, Fosun Pharma has the leading advantages in the market segments of blood circulation system, central nervous system, metabolism & digestive system, anti-infection, cardiovascular, oncology etc. With the brand concept of “innovation for good health”, Fosun Pharma is committed to becoming the world’s leading healthcare enterprise. In 2017 alone, Fosun Pharma had the revenue of ¥18.5 billion, ¥1.5 billion for R&D fund, and its market cap exceeded ¥100 billion. Fosun has been pursuing an international cooperative strategy, and has diversified & flexible investment cooperation models. For examples, Fosun Pharma acquired 74% stake in Gland Pharma for over $1 billion in 2017, and set up a joint-venture with Kite Pharma to develop and commercialize autologous T-Cell therapies to treat cancer, Yescarta is expected to become the first cell therapy product.
Leads by Mr. Yifang Wu, President and CEO of Fosun Pharma, the Bay Area promotional career forum hopes to have in-depth exchanges to discuss global cooperation and strategic blueprint with scientists, entrepreneurs and investors in the field of life sciences. Experts, students, scholars, entrepreneurs and investors with the interests of human medicine & healthcare are all welcome to attend.
上海复星医药集团成立于1994年,是中国领先的医疗健康产业集团, 业务覆盖医药健康全产业链,主要包括药品制造与研发、医疗服务、医疗器械与医学诊断、医药分销与零售。复星医药集团拥有国家级企业技术中心,打造了高效的化学创新药平台、生物药平台、高价值仿制药平台及细胞免疫平台, 目前,在血液系统、中枢神经系统、代谢及消化系统、抗感染、心血管、抗肿瘤等治疗领域均有产品在各自细分市场占据领先优势。复星医药以“持续创新 乐享健康”为品牌理念, 致力于成为全球主流医疗健康市场的一流企业. 2017年复星医药集团营收185亿人民币,研发投入15亿元,企业市值过千亿元人民币。2017年复星医药以10 亿美元收购Gland Pharma 74%股权, 与Kite Pharma合资共建复星凯特,一直秉承着国际合作战略,致力与国际领先企业合作共赢。
我们拥有多样灵活的投资合作模式
控股投资:
- 2017年10月3日完成对印度注射剂药品制造商GLAND PHARMA 74%股权的并购交易,推进集团的药品制造业务的产业升级、加速国际化进程。
- 收购欧洲呼吸机领先品牌Breas 80%股权,助力复星医药覆盖全球的线上线下一体化医疗器械运营平台的搭建。
BD引进:
2017年成功引进3个药物(Bremelanotide、卡巴拉汀贴片和Tenapanor)的中国商业化权力。
天使基金:
复星医药拥有多个天使基金平台专注于生命科技的前沿科学技术:SK-通德基金;以色列Pontifax基金;牛津OSI基金;硅谷BCF基金等
孵化器设立:
携手耶鲁大学许田教授,设立科技创新孵化平台,在全球范围内寻找、发现和培育创新项目。2017年12月,孵化平台运营实体复星领智已正式在上海注册成立。
合资公司设立:
- 携手美国Kite Pharma共建合营公司复星凯特,致力于研发和引进国际最先进的免疫细胞治疗技术,成为细胞治疗领域的创新型、规范化标杆企业。复星凯特已于2017年4月10日正式注册成立,落户于上海张江自由贸易试验区,并于2017年12月完成产业化基地建设。
- 与美国Intuitive Surgical启动双方在中国的战略合作,共同注资1亿美元在上海成立合资企业,研发、生产并销售基于机器人辅助导管技术、针对肺癌早期诊断治疗的创新产品。
新团队引进
- 投资成立重庆复创医药研究有限公司,引进王为波博士为公司总裁,组织领导小分子创新药的研究开发。复创公司已分别在上海张江和美国旧金山注册成立两个分公司,以充分利用全球新药研发资源。
- 与美国科学家团队合资组建上海复宏汉霖生物技术股份有限公司,致力于应用前沿技术进行单克隆抗体生物类似药、生物改良药以及创新单抗的研发及产业化,截至2017年12月31日,复宏汉霖已完成9个产品,16个适应症的IND申报,3 个项目进入临床III期,其中利妥昔单抗类似物预计于2018年上市。
- 以合伙人模式引入创新团队组建复星弘创,落户复星医药在苏州设立的创新研发中心,致力于抗肿瘤领域小分子创新药物开发。
- 以合伙人模式引入美国仿制药和微创新团队,致力于高价值仿制药及505b2产品开发。
复星医药一直坚持寻求与‘有企业家精神的科学家’建立长期合作”
---复星医药董事长陈启宇
此次湾区推介会由上海复星医药集团总裁兼CEO吴以芳先生带领,希望与湾区生命科学领域的科学家、企业家和投资界人士深入交流沟通,探讨全球化合作和战略布局。欢迎有志于人类医药健康领域的专家、学生、学者、企业家、投资界人士出席。

Yifang Wu
Executive Director of Fosun Pharma
President of Fosun Pharma
Chief Executive Officer of Fosun Pharma
Mr. Yifang Wu, joined Fosun in April 2004 and now serves as President and Chief Executive Officer of Fosun Pharma. Mr. Wu used to serve as a Technician, Director, Production Officer, Finance Director, Assistant to Director of Xuzhou Biochemical Pharmaceutical Factory, now known as Wanbang Pharma, from June 1987 to April 1997, Deputy Director of Xuzhou (Wanbang) Biopharmaceuticals Manufactures Plant, now known as Wanbang Pharma, from April 1997 to December 1998, President of Xuzhou Wanbang Biochemical Pharmaceutical Co., Ltd. , now known as Wanbang Pharma, Deputy General Manager of Wanbang Pharma from December 1998 to March 2007, President of Wanbang Pharma, from March 2007 to April 2011, and Chairman and CEO of Wanbang Pharma from April 2011 till now. Mr. Wu was Vice President and Chief Operation Director of Fosun Pharma from July 2014 to January 2016 and has been President and Chief Executive Officer of Fosun Pharma since June 2016, as well as Executive Director since August 2016.
吴以芳先生
复星医药执行董事
复星医药总裁
复星医药首席执行官
吴以芳先生于 2004 年 4 月加入复星,现任复星医药执行董事、总裁及首席执行官。
加入复星前,吴先生于 1987 年 6 月至 1997 年 4 月期间历任徐州生物化学 制药厂技术员、主任、生产科长、财务主任、厂长助理等职,于 1997 年 4 月至 1998 年 12 月期间任徐州(万邦)生物化学制药厂副厂长,1998 年 12 月至 2007 年 3 月任徐州万邦生化制药有限公司、江苏万邦生化医药股份有限公司副总经理(徐州生物化学制药厂、徐州(万邦)生物化学制药厂、徐州万邦生化制药有 限公司均为江苏万邦生化医药股份有限公司前身),于 2007 年 3 月至 2011 年 4 月期间任江苏万邦总裁,2011 年 4 月至今任江苏万邦董事长兼首席执行官。
吴以芳先生于 2014 年 7 月至 2016 年 1 月任复星医药高级副总裁,于 2016 年 1 月至 2016 年 6 月任复星医药高级副总裁、首席运营官,并于 2016 年 6 月至今任复星医药总裁、首席执行官,于 2016 年 8 月至今任复星医药执行董事。
Xuzhou Economic and Technological Development Zone 徐州经济技术开发区
Xuzhou Economic and Technological Development Zone lies in important strategic position of the "four vertical and four horizontal" system of the national railway network and is an important channel connecting the Beijing-Hangzhou Grand Canal Economical Belt and the Yangtze River Economical Belt. Founded in July 1992 covering an area of 293.6 square kilometers, Xuzhou Economic and Technological Development Zone has has upgraded to a state-level Economic and Technological Development Zone in March 2010. So far there are 4125 enterprises including 13 Fortune 500 companies in the zone. Currently Xuzhou Economic and Technological Development Zone’s comprehensive strength ranks 7th provincially of all 137 Development Zones in Jiangsu and has became a zone with largest scale, strongest strength, highest industry grade and of most investment value around Huaihai Economic Region.
徐州经济技术开发区位于全国铁路网“四纵四横”体系的重要战略位置,是连接京杭大运河经济带和长江经济带的重要通道。徐州经济技术开发区始建于1992年7月,面积293.6平方公里,已于2010年3月升级为国家级经济技术开发区。目前已有4125家企业入驻该区域, 其中包括13家世界500强企业。目前,徐州经济技术开发区综合实力位居江苏省137个开发区的第7位,已成为淮海经济区规模最大,实力最强,产业档次最高,投资价值最高的区域。

Weidong Jiang, Ph.D.
The company CSO and cofounder, Dr. Weidong Jiang, graduated from Zhejiang University with a bachelor degree, and Chinese Academy of Sciences, with a master degree. He later continued his Ph.D study in the University of Giessen in Germany and obtained a doctorate in Microbiology and Molecular Biology. After that, he conducted his post-doctoral research at University of California. Dr. Jiang has served as a senior scientist and director in companies like ChemGenics (Millennium), Microcide, Applied Molecular Evolution (Eli Lilly) and Catalyst Biosciences, with more than 20 years of experience in biopharmaceuticals development and production, including antibody/protein engineering, production cell line development, process optimization, and regulatory filing.
姜伟东博士
姜伟东博士,复宏汉霖联合创始人、高级副总裁兼首席科学官。姜博士曾先后在ChemGenics (Millennium),Microcide ,Applied Mol. Evolution (Eli Lilly) 及Catalyst Biosciences等公司担任高级科学家和总监职务,至今已有20多年的生技药品研发、生产经验,涉及抗体工程、高效重组基因表达系统工程、生产细胞株筛选及工艺优化, 药政等各个方面。姜博士曾就读于浙江大学, 于中国科学院上海细胞所获得硕士学位,后于德国吉森大学获得微生物与分子生物学博士学位,并在美国加州大学进行博士后研究工作。
Career Forum Figured Participating Companies:
1) Biocytogen Inc. headquartered in Beijing is one of the global leaders in gene targeted animial model production, preclinical in vivo efficacy studies and pharmacological service.
![]()
Opening positions (United States):
- In vivo efficacy/Toxicity Director
- PK/PD Scientists
- PK/PD Research Associates
- BD Director or Manager
2)Applied StemCell Inc. (ASC) is a fast growing biotechnology company headquartered in Milpitas, California. Our goal is to advance gene-editing and stem cell innovation for biomedical research and the biotechnology industry. After years of research and development, ASC is proud to offer an optimized series of tools for basic research study, drug discovery, bio-processing, bio-production and preclinical applications.
We are currently focusing on three areas: (1) cell line generation for bio-production and bioassays (2) patient-relevant cell models for personalized medicine and (3) physiologically predictive animal models of human diseases. We are striving to improve and expand our technologies and product lines to meet the increasing requirements of the biomedical community.

Opening positions (United States):
- Scientist in Molecular Biology and Cell Genome Editing
- Scientist in R&D Department
- Scientist in Product Development Department
- Scientist, Therapeutic Development
- Research Associate (R&D Department)
- Research Associate
- Research Associate, Therapeutic development
- Research Associate, Genome Editing/ Engineering
3) Kelun Pharmaceutical
- The Clinical Strategy Lead (CSL)
- Director, Early Stage Clinical Development
- Director, Late Stage Clinical Development
- Director, Clinical Operation Head
- Clinical Trials Liaison Officer (CTLO)
- Biologics Production Executive Director
- Director, Biologics Process Development
- Director of Biologics QA
- Director of Formulation Development
- Director of Generics Formulation(Solid Oral Products or Injectables)
4) Gilead
Gilead Sciences, Inc. is a research-based biopharmaceutical company focused on the discovery, development, and commercialization of innovative medicines.

There are 1200+ jobs opening including both R&D division and business positions. Details will follow soon.
5) Shanghai Henlius Biotech,Inc
Shanghai Henlius Biotech,Inc. is a joint venture company formed by Shanghai Fosun Pharmaceutical (Group) Co., Ltd. and Henlius Biopharmaceuticals Co., Ltd. in December 2009. The company focuses on the development, production and commercialization of mAb biosimilar drugs, bio-betters and novel mAbs using cutting-edge technology. Registered in Shanghai Zhangjiang, we have so far invested over 400 million RMB in the early development of monoclonal antibody drugs.
The concept of our drug development lies in global integrative innovation, and we have 3 R&D laboratories located in China (Shanghai), the US (California) and Taiwan (Taipei). Henlius is headquartered in the Shanghai Caohejing High-Technology Park. The total area of our Shanghai R&D laboratory is about 4,300m2, incorporating established development platforms of monoclonal antibody drugs. The company has completed the construction of a GMP standard facility with a 12,000 liter capacity, which adopted advanced disposable bioreactors based on the existing manufacturing facilities, ensuring the position of Henlius as a leading domestic antibody therapeutic testing and production site based in China.

Opening Positions (Shanghai,China):
- Commercial VP
- International Marketing Director/Manager
- Process Development VP/Director
- Production Manager
- Manufacture Director
- Engineering Dirctor
- Drug Safety Director/Manager
- Clinical Research Director/Manager, Oncology&Immunology
- Clinical Project Management Director/Manager
- CMC Project Director/Manager
- R&D Project Management Director/Manager
- Quality Assurance (QA) Director/Manager
- Quality Control (QC) Director/Manager
- Technical Services Director/Manager
- Business Development VP/Director/Manager
- Senior Scientist, Process Development
- Senior Scientist, Molecular and Cell Biology
- Regulation Affairs(RA) Manager
Opening Positions (Fremont,CA,US):
- Business Development VP/Director/Manager
- IP Attorney
- Senior Scientist, Process Development
- Senior Scientist, Molecular and Cell Biology
- Regulation Affairs(RA) Manager
More companies will be updated close to the event.
Tentative Event Schedule:
2:00 pm-2:30pm registration
Main Section:
2:30 pm - 2:40 pm Opening remarks: CABS, Dr. Cheni Kwok
2:40 pm – 3: 10 pm Keynote speech: Yifang Wu, President & CEO, Shanghai Fosun Pharmaceutical (Group) Co.,Ltd
3:10 pm - 3: 30 pm Dr. Weidong Jiang, CSO of Shanghai Henlius Biotech Inc
Career fair section:
3:30pm - 3:40 pm Biocytogen Inc.
3:40pm - 3:50 pm Applied StemCell Inc.
3:50pm – 4: 00 pm Gilead
Career fair and networking:
4:00 pm - 5: 30 pm
Schedule may be different on the day. Full job opening list and event detail will be released one week before event.
120 Li Ka Shing Center for Learning and Knowledge (LKSC), 291 Campus Drive, Stanford, CA 94305
Please register online. Online registration is free. Onsite registration is $10.
1:30 - 2:00PM check-in, onsite registration and networking
2:00PM - 4:00PM Seminars

Title of seminar: Molecularly Engineered Nanoparticles to Advance Personalized Diagnostics and Therapeutics
Aihua Fu, Ph.D. is a nanobiotechnologist and an entrepreneur. She is the co-Founder and CEO of NVIGEN Inc., a nanobiotech startup dedicated to developing and commercializing the best nanoparticles for biological research, diagnostic and therapeutic applications. Before she spearheaded the founding of NVIGEN with the support of serial biotechnology entrepreneur Dr. Guo-Liang Yu and other Silicon Valley investors, technologists and entrepreneurs, Aihua was trained as a postdoc at Stanford University Center of Cancer Nanotechnology Excellence under the supervision of Prof. Shan X. Wang and Prof. Sam S. Gambhir to engineer nanoparticles with enhanced properties for in vitro diagnostics and in vivo cancer imaging and drug delivery applications. Aihua received her Ph.D. from the Chemistry Department of University of California at Berkeley with Prof. Paul Alivisatos, which built a solid foundation for her understanding of the unique properties of nanoparticles and their huge potential in helping define personalized disease diagnostics and therapy. Her nanoparticle research and product development efforts were also supported by NIH Pathway to Independence Award and NIH SBIR Awards.
NVIGEN Overview. NVIGEN has developed a platform of molecularly engineered nanoparticles that can be tailored and automated to enable a broad range of diagnostic and therapeutic products advancing the personalized medicine revolution. The particles have been engineered 1) to capture nucleic acids, proteins, and cells with a high degree of sensitivity and specificity, enabling low-cost, highly accurate diagnostics, or 2) to enhance the efficacy of therapeutics by providing targeted drug delivery or better T-cell activation and expansion for more effective cell therapy. NVIGEN holds and has exclusive rights to 7 patents, with an additional 11 patents pending, covering compositions of matter and a wide range of diagnostic and therapeutic applications.

Title of seminar: Molecular Imaging in the Era of Precision Medicine
Dr. Zhen Cheng is an Associate Professor of Radiology and a member of the Molecular Imaging Program at Stanford (MIPS), BioX program, Canary Center at Stanford for Cancer Early Detection and Stanford Cancer Center. He currently leads the Cancer Molecular Imaging Chemistry Laboratory of the MIPS where he is developing novel molecular probes, non-invasive imaging techniques and bionanotechnology for the multimodality early detection of cancer.
Dr. Cheng has published over 200 peer reviewed articles (From Google Scholar, H-index: 56; Citation >10600), 9 book chapters, and he is a co-inventor of 14 patents. Dr. Cheng is the immediate past President of Chinese American Society of Nuclear Medicine and Molecular Imaging, and he was the Board Director of the Radiopharmaceutical Sciences Council in the Society of Nuclear Medicine and Molecular Imaging. He has served as a reviewer for numerous funding agencies including NIH, DOD, DOE, German Research Foundation, National Natural Science Foundation of China, the Agency for Science, Research & Technology (A*STAR) in Singapore, etc. He has also served as an active reviewer for over 100 research journals such as Nature Nanotechnology, Nature Communications, Science Translational Medicine, JACS, Angewandte Chemie, JNM, etc., abstract reviewer and session chair for numerous meetings (SNM, WMIC, SRS, etc.). Moreover, he is Associate Editor of Frontiers in Biomedical Physics; Nanomedicine: Nanotechnology, Biology and Medicine and sits on the editorial board of numerous peer-reviewed journals including Clinical Cancer Research, Bioconjugate Chemistry, Amino Acids, etc.
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Nature Jobs Career Expo will be in Los Angeles (3/31) and in San Francisco (4/2). See details below.
March 31,2018 LA 9:00am-3:30pm
Palisades Room at Carnesale Commons
251 Charles E Young Drive West, UCLA
Los Angeles California CA 90095
April 2,2018 SF 9:00am-3:30pm
Pauley Ballroom, Martin Luther King Jr. Building
2495 Bancroft Way, University of California, Berkeley
San Francisco California CA 94720

2495 Bancroft Way, University of California, Berkeley

Please RSVP, the registration will be closed on 11:59 AM, March 28, 2018.
Please register here
Title: Genome-scale signatures of gene interaction from compound screens predict clinical efficacy of targeted cancer therapies

Dr. Peng Jiang is a postdoc research fellow at Shirley Liu Lab at Dana Farber Cancer Institute and Harvard School of Public Health. Peng finished his Ph.D. at the Lewis Sigler Genomics Institute at Princeton University, and he finished his undergraduate study with the highest honor at the department of computer science at Tsinghua University. Peng's research focused on developing computational models to identify biomarkers and regulators of anticancer drug resistance. Recently, Peng developed CARE, a computational method focused on targeted therapies, to infer transcriptomic signatures of patient clinical response from cellular compound screens (Jiang et al., Cell Systems 2018). The CARE model on targeted therapy efficacy can also be applied to determine the gene biomarkers of cancer immunotherapy response and resistance (Jiang et al., Nature Medicine in Revision). Peng also did many influential works on large-scale cancer data integration and biological network analysis. For example, the ENCODE consortium utilized his algorithm RABIT (Jiang et al., PNAS 2015) on the analysis of ENCODE3 genomics data and identified SUB1 as a new RNA binding protein in promoting tumor progression (Nature under review, Jiang as co-first author). Peng also developed a highly efficient network clustering algorithm SPICi, which has over 160 citations (Jiang et al., Bioinformatics 2010).
Abstract
Identifying reliable drug response biomarkers is a significant challenge in cancer research. We present CARE, a computational method focused on targeted therapies, to infer transcriptomic signatures of drug efficacy from cell line compound screens. CARE outputs genome-wide scores to measure how the drug target gene interacts with other genes to affect drug efficacy in the compound screens. When evaluated using transcriptome data from clinical studies, CARE can predict the therapy outcome better than signatures from other methods. Moreover, the CARE signatures for the BRAF inhibitor are associated with an anti-PD1 clinical response, suggesting a common efficacy signature between targeted therapies and immunotherapies. When searching for genes in lapatinib resistance, CARE identified PRKD3 as the top candidate. PRKD3 inhibition, by both siRNA and compounds, significantly sensitized breast cancer cells to lapatinib. Thus, CARE should enable large-scale inference of response biomarkers and drug combinations for targeted therapies using compound screen data.
Online
Genome-Scale Signatures of Gene Interaction from Compound Screens Predict Clinical Efficacy of Targeted Cancer Therapies
Time: 03/30/2018 Friday, 12:00 PM (EST) to 1:00 PM (EST) (9:00 AM - 10:00 AM PDT)
In-person seminar location: Longwood Center | 360 Longwood Ave., Boston, MA, USA (in-person attendance is welcome!)
Webinar link will be sent 24 hours before the event.
RSVP by 11:59 AM PDT, March 28, 2018 using the link below:
|
In-person session is located here: Longwood Center | 360 Longwood Avenue, Boston, MA, United States If you are located in Boston area, please come to our in-person session if convenient. The time is 03/30/2018 Friday from 12:00 PM (EST) to 1:00 PM (EST). The paper for this talk: Peng Jiang, Winston Lee, Xujuan Li, Carl Johnson, Jun S. Liu, Myles Brown, Jon Christopher Aster, and Xiaole Shirley Liu. In Press. “Genome-scale signatures of gene interaction from compound screens predict clinical efficacy of targeted cancer therapies.” Cell Systems. Time: 9:00 AM - 10:00 AM PDT (12:00 PM - 1:00 PM EST), Friday, March 30, 2018 Zoom.us link will be sent 24 hours before the event. Please RSVP, the registration will be closed on 11:59 AM, March 28, 2018. Abstract Identifying reliable drug response biomarkers is a significant challenge in cancer research. We present CARE, a computational method focused on targeted therapies, to infer transcriptomic signatures of drug efficacy from cell line compound sc |
The paper for this talk: Peng Jiang, Winston Lee, Xujuan Li, Carl Johnson, Jun S. Liu, Myles Brown, Jon Christopher Aster, and Xiaole Shirley Liu. In Press. “Genome-scale signatures of gene interaction from compound screens predict clinical efficacy of targeted cancer therapies.” Cell Systems .
Abstract
Identifying reliable drug response biomarkers is a significant challenge in cancer research. We present CARE, a computational method focused on targeted therapies, to infer transcriptomic signatures of drug efficacy from cell line compound screens. CARE outputs genome-wide scores to measure how the drug target gene interacts with other genes to affect drug efficacy in the compound screens. When evaluated using transcriptome data from clinical studies, CARE can predict the therapy outcome better than signatures from other methods. Moreover, the CARE signatures for the BRAF inhibitor are associated with an anti-PD1 clinical response, suggesting a common efficacy signature between targeted therapies and immunotherapies. When searching for genes in lapatinib resistance, CARE identified PRKD3 as the top candidate. PRKD3 inhibition, by both siRNA and compounds, significantly sensitized breast cancer cells to lapatinib. Thus, CARE should enable large-scale inference of response biomarkers and drug combinations for targeted therapies using compound screen data.
--
Longwood Center | 360 Longwood Ave., Boston, MA, USA
If any company is interested in sponsoring, please contact sponsorship@cabsweb.org.
When register, please enter your affiliation and name on your photo ID which is required at onsite check-in.
Please register online. Online registration is free. Onsite registration is $10.
1:30 - 2:00PM check-in, onsite registration and networking
2:00PM - 4:00PM Seminars

Title of seminar: Hepatocyte stem cells as cell replacement therapy for liver disease
Bruce Wang, MD, is an Assistant Professor at Department of Medicine, Division of Gastroenterology, UCSF. The long-term goals of Bruce’s lab are to improve the understanding of liver disease pathophysiology and develop novel methods of treatment for liver diseases, including cell replacement therapy. Currently, we have two major research focuses: 1) understanding the biology of adult hepatocyte stem cells and 2) developing a liver cell atlas.
Bruce was born in China and grew up in the San Francisco Bay Area. He went to college at Stanford and did his medical school, residency and fellowship at UCSF. After a postdoctoral fellowship at Stanford, he came back to UCSF to start his lab. He enjoys exploring and photographing the Bay Area outdoors with his wife and two young sons.

Title of seminar: Stem Cells and Genomics for Precision Medicine
Joseph C. Wu, MD, PhD is Director of the Stanford Cardiovascular Institute and Simon H. Stertzer Professor of Medicine (Cardiology) and Radiology at the Stanford School of Medicine. Dr. Wu received his MD from Yale University School of Medicine. He trained in internal medicine and cardiology at UCLA followed by a PhD in the Dept of Molecular Pharmacology.
Dr. Wu has published >300 manuscripts. His lab works on biological mechanisms of patient-specific and disease-specific induced pluripotent stem cells (iPSCs). The main goals are to (i) understand basic cardiovascular disease mechanisms, (ii) accelerate drug discovery and screening, (iii) develop “clinical trial in a dish” concept, and (iv) implement precision cardiovascular medicine for prevention and treatment of patients. His lab uses a combination of genomics, stem cells, cellular & molecular biology, physiological testing, and molecular imaging technologies to better understand molecular and pathophysiological processes.
Dr. Wu is an Associate Editor for Circulation Research and Senior Guest Editor for JACC: Cardiovascular Imaging. He is also on the editorial board of Journal Clinical Investigator, Circulation Cardiovascular Imaging, Human Gene Therapy, Molecular Therapy, Stem Cell Research, Journal of Nuclear Cardiology, Physiological Genomics, Scientific Report, and Nature Review Cardiology.
Dr. Wu has received numerous awards, including National Institutes of Health (NIH) Director’s New Innovator Award (2008), NIH Roadmap Transformative Award (2009), American Heart Association (AHA) Innovative Research Award (2009), Presidential Early Career Award for Scientists and Engineers given out by President Obama (2010), AHA Established Investigator Award (2012), Burroughs Wellcome Foundation Innovation in Regulatory Science Award (2015), and AHA Merit Award (2017). He received the Best Basic Science Manuscript Award in Circulation (2006 & 2014), Best Manuscript Award in Circulation Research (2013 & 2017), and the William Parmley outstanding paper award in JACC (2009 & 2017). He also received the inaugural AHA Joseph A. Vita Award (2015) which is given to an investigator whose body of work published in the last 5 years has had transformative impact on basic, translational, or clinical cardiovascular research. Dr. Wu currently serves on the Scientific Advisory Board for the Keystone Symposia (2014-2020), FDA Cellular, Tissue, and Gene Therapies Advisory Committee (2017-2020), AHA National Board of Directors (2017-2019), and Chair of the AHA National Research Committee (2017-2019).
Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080
Visit the BioPacific Toastmasters during our special meeting with two guest speakers:
- Michael Chong, ACG, the winner of the International Speech Contest at his club, Andeesheh Toastmasters, will open us to a whole new world of public speaking. He will inspire us with a speech on "You Don't Have To Be Great To Start" that enables us to envision his vivid journey.
- Tim Carlisle, DTM, is a Distinguished Toastmaster, who will guide us on Pathways. He will help us register online, pick a path, and navigate through Base Camp.
To elevate your thinking, communication, and leadership skills to the next level, learn how Toastmasters International can help develop your personal life and professional career with mentors and buddies:
- Scientists can break the ice and enrich presentations by researching topics and organizing speeches with visual aids that entertain and inspire audiences,
- Entrepreneurs can practice getting to the point and telling stories when pitching how their technology transforms industries, and
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language.
Let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun at weekly in-person and virtual meetings! Here is a photo from our first speech contest where contestants receive a FREE code (worth $200) for a presentation and public speaking course by Chris Haroun, an award-winning professor and venture capitalist:

For more info, refer to the following:
- Website: http://biopacific.toastmastersclubs.org
- Newsletters: editions in 2018
- Email: contact-6480331@toastmastersclubs.org
Photo and video disclaimer:
- Portions of this event will be recorded with photos and videos
- Your ticket purchases implies consent to this recording for the publicity of Toastmasters International and its affiliates (e.g., BioPacific Toastmasters), without financial compensation or editorial control
1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010
Event: BioDuro Symposium - Innovative Approaches to Accelerating Drug Discovery and Development
Registration Link: https://bioduro.com/landing/symposium-sf-2018/
How can we accelerate successful drug discovery and development?
The success of clinical candidate development depends on several factors, important among them are generation of novel, therapeutically-relevant chemical structures, early and rapid assessment of possible efficacy of candidate structures in pre-clinical therapeutic models and rapid elimination of candidates that will ultimately prove unsuccessful. Innovative processes and techniques that facilitate optimal candidate selection is a crucial step in accelerating drug discovery and development timelines.
Join us on Thursday, March 22, 2018 for an afternoon of lunch, insightful presentations and discussion, followed by a networking and happy hour reception.
BioDuro invites you to hear from industry experts on:
From Small Fragments to Big Drugs: Fragment-based Drug Discovery
Dan Erlanson, Ph.D | Co-founder and VP of Chemistry | Carmot Therapeutics
From Fragments to Drugs: Playing Quick Molecular Scrabble with Protein Crystallography
Debanu Das, Ph.D | Co-founder and CEO | Accelero Biostructures
Role of DMPK in Drug Discovery and Development
JP Shaw, Ph.D | Independent Pre-Clinical DMPK Consultant
Paving the Road to the Clinic: Advancing Oncology Drug Development with Translational In Vitro and In Vivo Models
Tommy Broudy, Ph.D | CEO | Molecular Response
Non-Toxic Pre-Clinical Formulations for Insoluble Compounds: Applying Enabled Formulations Approaches to Late Phase Discovery
Blair West, Ph.D | Chief Science Officer, Global CMC Solutions | BioDuro
Rapid Development and Scale-up of Solubilized Dosage Forms
Ruchit Trivedi, Ph.D | Associate Director, Formulation | BioDuro
We look forward to seeing you in San Francisco!
Agenda:
Registration/Lunch
Speakers
Networking Happy Hour
Registration for this event is free, but space is limited. RSVP here!
Hilton Garden Inn, South San Francisco
Visit the BioPacific Toastmasters during our International Speech Contest where contestants and a guest speaker will deliver inspiring speeches. Pradeep Gandhi, the winner of the contest at his club, Intuitively Speaking, will open us to a whole new world of public speaking by guiding us with a speech on the "North Star to Forgiveness" that enables us to envision his vivid and an emotional journey.
To elevate your thinking, communication, and leadership skills to the next level, learn how Toastmasters International (TI) can help develop your personal life and professional career with mentors and buddies:
- Scientists can break the ice and enrich presentations by researching topics and organizing speeches with visual aids that entertain and inspire audiences
- Entrepreneurs can practice getting to the point and telling stories when pitching how their technology transforms industries
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language
Let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun at weekly in-person and virtual meetings! Here is a photo from our first speech contest where contestants receive a FREE code (worth $200) for a presentation and public speaking course by Chris Haroun, an award-winning professor and venture capitalist:

For more info, refer to the following:
- Flyer: International Speech Contest
- Website: http://biopacific.toastmastersclubs.org
- Email: contact-6480331@toastmastersclubs.org
Photo and video disclaimer:
- Portions of this event will be recorded with photos and videos
- Your ticket purchases implies consent to this recording for the publicity of Toastmasters International and its affiliates (e.g., BioPacific Toastmasters), without financial compensation or editorial control
1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010
Visit the BioPacific Toastmasters during our Table Topics Contest where contestants respond to the same topic with an impromptu speech. To elevate your thinking, communication, and leadership skills to the next level, learn how Toastmasters International (TI) can help develop your personal life and professional career with mentors and buddies:
- Scientists can break the ice and enrich presentations by researching topics and organizing speeches with visual aids that entertain and inspire audiences
- Entrepreneurs can practice getting to the point and telling stories when pitching how their technology transforms industries
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language
Let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun at weekly in-person and virtual meetings! Here is a photo from our first speech contest where contestants receive a FREE code (worth $200) for a presentation and public speaking course by Chris Haroun, an award-winning professor and venture capitalist:

For more info, refer to the following:
- Flyer: Table Topics Contest
- Website: https://biopacific.toastmastersclubs.org
- Email: contact-6480331@toastmastersclubs.org
Photo and video disclaimer:
- Portions of this event will be recorded with photos and videos
- Your ticket purchase implies consent to this recording for the publicity of Toastmasters International and its affiliates (e.g,. BioPacific Toastmasters), without financial compensation or editorial control
1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010
The success of an Investigational New Drug (IND) application is one of the key milestone achievements of the drug development process. WuXi AppTec’s Laboratory Testing Division is pleased to invite you to an in-depth look into the WIND program, an integrated service platform for your IND project. We will help you Win your IND! Please join WuXi’s workshop to learn more details on basic requirements, study design, project implementation, program management, dossier preparation and regulatory strategy for a successful IND application.
https://www.eventbrite.com/e/wind-in-san-francisco-2018-tickets-42544068454
Crowne Plaza - Foster City Hotel 1221 Chess Drive Foster City, CA 94404
Registration: Free
Registration Limit: 150 people
Description: In collaboration with CKR Law, we would like to cordially invite everyone to attend a workshop and panel discussion on cross border investments and M&A to be held on February 28th. Speakers will include successful entrepreneurs, M&A attorneys, and life science investors. Topics covered will include the healthcare start-up scene in China, CFDA registration process, legal structures for entering the China market, and more. Anyone with an interest in entering the Chinese market or raising Chinese capital / collaboration in the United States will derive tremendous value from this event.
This workshop will be open for registration to everyone and is expected to fill up quickly, so register today to reserve your spot.
•Agenda:
•2:00-2:30 — Networking
•2:30-3:00 — Medical device regulations in China and the latest approaches
Speaker: Yongheng Chang
•3:00-3:30 — From science to health, an entrepreneur’s experience
Speaker: Guoliang Yu
•3:30-4:00 — The healthcare market and the environment for entrepreneurs in China
Speaker: Victor Shi
•4:00-4:30 — CFDA registration of medical devices and pharmaceuticals and the trial program with Shanghai FTZ
Speakers: Wei Zhang, Partner of CKR Law, Rong Sun, Counsel of CKR Law
•4:30-4:45 — Possible legal structures to enter into China market as a Chinese domestic company
Speaker: Ning Zhang, Partner of CKR Law (Chair of China Practice, West Coast)
•4:45-5:30 — Panel discussion (The investment outlook on cross-border biotechnology industry in 2018)
Moderator: Xu Chen
Panel members:
Tony Chen, Partner of Jones Day
Lishin Wang, CEO of Bondent Dental
Vincent Xiang, 6 Dimensions Capital
Wendy Shao, Investment & M&A, Bio-Techne Co.
Leslie Overman, Partner, CKR Law
•5:30-6:00 — Q&A and Network

Mr. Chang Yongheng, Executive Chairman of the China International Medical Device Regulatory Forum (CIMDR) -- the world’s largest annual medical device regulatory conference held by China Center for Food and Drug International Exchange (CCFDIE) of the China Food and Drug Administration (CFDA). He is also Deputy Director of the Committee for Research of Medical Device Regulation of the China Society for Drug Regulation (CSDR), Deputy Director of the Committee for Research of International Exchange and Cooperation of CSDR, Senior Member of the Chinese Pharmaceutical Association, and Chief Adviser of Ramed Bioscience Consulting, LLC.
His career started with 7 years in a medical device manufacturing company and 4 years in a pharmaceutical company. Following that, he worked in government sector for 30 years, being a Deputy Division Director in the Department of International Cooperation of SPAC (State Pharmaceutical Administration of China), Director of the Foreign Product Registration Division of the Department of Medical Devices of SPAC, Director of the Product Registration Division of the Department of Medical Devices of SFDA (State Food and Drug Administration), Deputy Counsel of the Department of Medical Devices of SFDA, and Deputy Director General of CCFDIE of CFDA successively.
His paper “Look at the Issues in the Distribution and Use of Medical Products in China with a People Oriented View” was published on “National Medical Information”, “Food and Drug Development and Administration”, “China Food and Drug Administration” and “Chinese Journal of Medicinal Guide” during 2005 - 2006. It was adopted into the large theoretical literature of “Great Practice in Implementation of the Scientific Development Concept” and appraised as the Excellent Theoretical Achievement by the China National School of Administration in 2006. Mr. Chang was rewarded with a Certificate of Honor and appointed as a Special Editor by the China National School of Administration at the same time.
Many of his papers on supervision of medical devices can be found in different journals such as the “Chinese Journal of Medicinal Guide”, “China Food and Drug Administration”, “China Medical Device Information”, “China Medical Device Journal”, “China Laboratory Medical Supplies” and “World Medical Devices”.

Dr. Guoliang Yu, Ph.D., serves as the Chairman at Jiangsu Quanxin Biopharmaceutical Co., Ltd. Dr. Yu founded QuanDx Inc. in 2010. He Co-Founded Epitomics Inc. in 2002. Dr. Yu is the Co-Founder of CBT Pharmaceuticals, Inc and serves as its Chairman of the Board. He serves as Executive Chairman of Crown Bioscience, Inc. and has been its Chief Technology Officer since August 18, 2016. Dr. Yu serves as Venture Partner at OrbiMed Advisors, L.L.C. He serves as a Consultant at Efung Capital. Dr. Yu served as the Chairman, Chief Executive Officer and President of Epitomics, Inc. He served as Senior Vice President of Research and Development at Mendel Biotechnology Inc. At Mendel Biotechnology, Dr. Yu established a robust functional genomics program, exceeded all six milestones for the Research Agreement with Monsanto and Savia and managed over 60 Ph.D. scientists and research associates. Dr. Yu worked with Human Genome Sciences, Inc., as one of the first Senior Scientists involved in the establishment of the high throughput gene discovery program. While at Human Genome Sciences, he led the technology development group and cancer group. Dr. Yu identified and characterized numerous genes for therapeutic protein and drug discovery. He contributed to the discovery of BLys. He then joined Dr. Frederick Ausubel's lab at Harvard to pursue the question how plants defend themselves against pathogens without an immune system. Dr. Yu identified the first plant disease resistance gene. In 1993, in stead of continue his academic advancement. In 1998, he was attracted to identify plant genes with economic value in agriculture and in bio-energy. Dr. Yu serves as Member of Advisory Board at DiaCarta Inc. He serves as Director at SISCAPA Assay Technologies, Inc. He serves as a Director of Applied StemCell Inc. Dr. Yu serves on the boards of several professional organizations including Bayhelix, CABS, National Foundation of Cancer Research, Chinese Biopharmaceutical Association, Ray Wu Memorial Foundation, University of Pacific in the US and China. He has more than 12 years of experience in genomics and 24 years of experience in life sciences research. Dr. Yu is involved in coaching young entrepreneurs and also serves on the board of directors for six start-up companies. He is Co-Inventor of more than 200 patents and Co-Author of over 100 patents and scientific articles and has Co-Authored 40 peer-reviewed scientific articles. Dr. Yu is the Founder of the Chinese Biopharmaceutical Association. He is one of the experts for the China's "1000 Plan."Dr. Yu was a Post-Doctoral Fellow at the Harvard Medical School. He holds a Ph.D. degree in Molecular Biology from the University of California, Berkeley and Indiana University and a B.S. degree in Biochemistry from Fudan University, Shanghai, China in 1984.

Dr. Victor Shi is Chairman of the BayHelix Group, a global association of Chinese life science business executives. Since 2017, he is Managing Partner of Serica Capital, focusing on investing in IVD and medical devices in China. Meanwhile, he is Founding President Asia Pacific of QIAGEN, a leading player of In-Vitro diagnostics and life science research service provider home and abroad.
Dr. Shi has 25 years’ experience in life science R&D, sales and marketing, business development, and investment. Previously-held positions include Managing Partner, Aura Partners, SVP of Corporate Development, Bridge Pharmaceuticals, VP of Corporate and Business Development, Genospectra (later Panomics) and Director of Asia Operations at A. M. Pappas & Associates. He was also a faculty member at National University of Singapore School of Medicine and a cancer research scientist at Merck & Co.
He is currently Vice Chair of AdvaMed China Board and Chair of its IVD Working Group. Meanwhile, he is Deputy Secretary-General of the Professional Committee of Chinese In-Vitro Diagnostics Industry Association.
Dr. Shi holds Ph.D. and M.S. degrees from the University of Rochester, New York, and a B.S. degree from the University of Science and Technology of China.

Dr. Vincent Xiang is a Partner of 6 Dimensions Capital. He has over 20 years of experience in the life sciences industries in the U.S. and China. From 2013 to 2016, Dr. Xiang was Managing Director and Head of international investments & business development at Humanwell Healthcare Group (600079.SH), which has $1.3B/year in revenue in 2015. He led the $550M acquisition of US Epic Pharma, the largest M&A ever done by a Chinese healthcare firm. Prior to that, he worked as Managing Director of Burrill Life Science Venture Capital Fund, scouting first in class assets. From 2004 to 2012, Dr. Xiang was Portfolio Manager/Analyst at Franklin Templeton, investing in global life science companies at all stages. Previously, he was Vice President of business development at Genyous, a US biotech start-up. He was also Director of venture investment at Acacia Research, Associate at BioAdvance (a healthcare Angel fund), and Irvington Fellow at Skirball Center and licensed his patented technology to Millennium Pharmaceutical for royalty payments. Dr. Xiang began his career at Sinopharm in China.
Dr. Xiang received his Ph.D. in molecular biology from University at Stony Brook, MBA from the Wharton School, and B.S. in Immunology and Microbiology from Fudan University. He is a founding member of BayHelix Group, founders and board members of two life science startups.

Tony Chen is a partner at Jones Day Shanghai office.
Tony Chen represents clients for their cross-border intellectual property and life sciences legal issues. He helps multinational companies reduce IP risks and enforce their IP rights in civil and criminal actions in China and represents Chinese companies in building and procuring intellectual property on a worldwide basis. Tony co-chairs the IP subcommittee of the American Chamber of Commerce in Shanghai and serves on the expert panel of the US – China IP Cooperation Dialogue. He has advised Shanghai Pudong District and Suzhou BioBay on the development of biopharmaceutical industry.

Mr. Lishin Wang serves as the CEO of Bondent Technology Corp, a dental equipment start up based in China. He joined the company in 2016 and is working diligently with the team to bring this local company to IPO through local expansion and global M&A.
Mr. Wang has worked in the global health care industry for 28 years with extensive experiences ranging from R&D to Sales and Marketing. He started his career in 1989 as a research scientist in the field of laser surgery, and gradually moved his career path to business management. He joined Johnson and Johnson Medical Asia Pacific in 2000 and held several senior country and regional roles, including e-Business Director of Asia Pacific, General Manager of IVD business in China, Managing Director of J&J Medical Taiwan, and North Asia Cardiology Business Director. In 2011, Mr. Wang was recruited by DAKO, a PE funded tissue based cancer diagnostic company, as the Corporate VP of Asia Pacific. The company was later acquired by Agilent in 2013. After leaving Dako, Mr. Wang joined Leica Microsystems China, A Danaher Life Science Company as the General Manager until 2016.
Mr. Wang holds an MBA degree in Finance from The Wharton School and an MS degree in Applied Physics from Georgia Institute of Technology. He also holds a US patent in photon color printing.

Dr. Shao is in charge of NASDAQ listed company Bio-Techne’s merger and acquisition and investment activities in Asia. For the past years, Bio-Techne had at least 4-5 acquisitions every year, involving companies in both China and the United States. Dr. Shao built Bio-Techne's China business ten years ago. Annual sales of Chinese operations grew from zero to RMB300 million. With the acquisition of a local Chinese firm, Bio-Techne China has fully integrated research & development, customized service and GMP manufacturing capabilities in China.
Prior to her return to China, Dr. Shao served as the director of M&A and investment of medical devices business at Guidant & Abbott Laboratories with experiences of approximately $30 billion of M&A and investment deals. She was responsible for license negotiation and intellectual property acquisition for drug stents. The global annual sales of drug eluting stent now reached more than 6 billion U.S. dollars.
Dr. Shao also oversaw early investments projects such as fully absorbable stents, cell therapies and heart valves. Many of these projects had been listed publicly or successfully exited. The $27 billion BSX/GDT/ABT deal was the largest Wall Street deals at that time. Before joining Abbott, Dr. Shao worked for Hewlett-Packard and took part in the spin-off HP's Medical Devices and Testing Group as Agilent. Agilent successful went public on NYSE and raised 2.1 billion U.S. dollars. It was the largest IPO of the year.

Ning specializes in cross-border transactions. In her close to ten-year legal career, she spent half of her time in China and half in the U.S. helping clients investing in China or U.S. through green field investment, mergers and acquisitions, joint ventures, PE/VC financing, among others. She is also experienced with commercial bank transactions in the U.S.
Selected Transactions:
•Mergers and Acquisitions
Represented a major U.S. high tech company in strategic acquisitions of various companies or assets, involving deep learning, cloud technology, CDMA business, etc.
Represented Google Capital in a proposed acquisition of an online education site in China (not closed)
Represented Hepalink, a Chinese company listed on Shenzhen Stock Exchange, in its acquisition of its competitor in the U.S. and its investment in a medical fund in the U.S.
Represented ICBC in its acquisition of a Taiwan commercial bank
Represented owners of a Chinese hospital restructure its business in a sale to a British strategic investor
Represented and participated in a number transactions where the U.S. investors disposed their interests in Chinese entities
•PE/VC Financing Deals
Represented certain medical fund in China to invest in various U.S. companies in Pre-IPO rounds
Represented a well-known U.S. pharmaceutical company in its investment in Chinese pharmaceutical companies through its fund in Asia
Represented Chinese investors in Series F investment in AirBnB and Uber
•Joint Ventures
Participated in about ten joint ventures or strategic alliance projects in various jurisdictions, including Hong Kong, Singapore, China, Cayman Islands, BVI and U.S.
•Commercial Banks
Assisted ING to form its representative office in the U.S.
Participated in more than 20 commercial banks' debt issuance or public offerings

Wei has a decade of experience in practicing intellectual property law. He advises clients on the management of U.S. and foreign IP portfolios, as well as freedom to operate, invalidity and non-infringement issues. Wei has represented clients in patent infringement and commercial litigations in the U.S. Additionally, Wei performs due diligence investigations in connection with acquisition, licensing, and technology transfer transactions, and drafts commercial agreements.
Wei was selected to the list of New York-Metro rising star for intellectual property law by Super Lawyers in years 2014-2017.
Selected Matters:
• Managed global patent portfolios for clients spanning from multinational corporations, research universities to start-ups, including Celgene Corp., Genentech, Inc., Boston Scientific Corp., etc.; and advised clients on various regulatory compliance matters.
• Represented Acorda Therapeutics, Inc. in the acquisition of a privately-held biotechnology company.
• Represented Celgene Corp. in the acquisition of a biopharmaceutical company.
• Represented Texas Instruments Inc. in a royalty obligation dispute pursuant to a patent portfolio license agreement.
• Represented a Taiwanese company in a patent infringement action relating to display technology.
• Represented a major U.S. pharmaceutical company in a patent invalidation action in Chinese patent office and courts.
• Represented a number of U.S. companies in patent interference, inter parte review, and opposition actions in the U.S., Europe, Japan, Korea, and Mexico.
Represented several start-up companies in licensing and joint venture transactions.
With decades of medical practice and hospital management experiences as well as solid legal knowledge and expertise, Ms. SUN is specialized in providing legal service to corporations and organizations of medical and pharmaceutical industry with regard to investment, incorporation, merge and acquisition and transformation.
Ms. SUN acts as outside legal counsel for a wide range of leading corporations in pharmaceutical and medical service industry, including Everjoy Health Group (SC002162), Town Health International Medical Group(HK3886), Malo Clinic Group, Beijing Cuiyutao Children’s Healthcare Center, Landseed Medical Group, Suzhou Symap Medical Equipment Co. Ltd., Suzhou Park Medical Co. Ltd., , Shanghai Yuecheng Medical Investment and management Co. Ltd.(a wholly-owned subsidiary of CITIC PE), Shanghai Wenyi Hospital, Shanghai Chinese Medicine and Clinic Co. Ltd and Optegra Eye Health Care.
Ms. SUN participates in many joint venture establishment projects in medical service area, and some cases are pioneering in China’s medical service market. These projects include establishment and incorporation of Shanghai Guang Ci Memorial Hospital, Rui Li Plastic Surgery Hospital, Shanghai Rui Dong Hospital (Now named Parkway Health), Cascade Healthcare.
Ms SUN also possesses extensive experience in M&A, corporate restructuring and financing. She has provided legal services to many companies for their investment and M&A projects, such as the series A and B funding of Beijing Cuiyutao Children’s Healthcare Center, Malo Clinic Group, Suzhou Symap, and Serie A funding of Suzhou Park Medical.
Thanks to alliance organizations and sponsors

Hanqi (1633 Old Bayshare Hwy, Suite 280, Burlingame, CA 94010)
Matching will be based on a variety of factors such as background, mutual interest, locations, so it is not guaranteed. We will officially launch the 2018 CAN program in April (Kick-off meeting date TBD). Here is a tentative schedule for this year’s program.
- Mentor/Mentee registration opens: Fri, 2/23/2018
- Registration closes: Fri, 3/16/2018
- Program acceptance, email notification sent: 1st week of Apr
- Kick off event: Late Apr
- Mentoring groups convene: May-Oct
- Graduation: Nov
Please visit http://www.cabsweb.org/can/ to learn more about the CAN program. If you are interested in becoming a mentor or mentee, please complete the forms below by 5pm on Fri, 3/16/2018.
- MENTOR registration: https://goo.gl/forms/3FxYmfOV4gCN9OHS2
- MENTEE application: https://goo.gl/forms/CCHbHblCgkdPFhFy1
Online
|
2018 CABS Chinese New Year Party Happy Chinese New Year Year of the Dog The old year leaves amidst the falling snow 瑞雪纷飞辞旧岁 The new spring comes with the shining glow 旭日东升迎新春
Please mark your calendar and don’t forget to register online. Please also specify the numbers of Children and Seniors whom you will bring in the "Comments" field. This will help us prepare the right amount of food. Boxed lunch may not available if not registered on line!!! We look forward to seeing you there!
|
Auditorium, Sofia University, 1069 E Meadow Cir,Palo Alto, CA 94303
Visit the BioPacific Toastmasters during our Open House to learn the key to success for overcoming procrastination with a motivational speech by Keh Chang Huang, a past Division Director of District 4. Celebrate the lunar new year with us and refreshments (before registering HERE for the party hosted by the Chinese American Biopharmaceutical Society). To elevate your thinking, communication, and leadership skills to the next level, learn how Toastmasters International (TI) can help develop your personal life and professional career with mentors and buddies:
- Scientists can break the ice and enrich presentations by researching topics and organizing speeches with visual aids that entertain and inspire audiences
- Entrepreneurs can practice getting to the point and telling stories when pitching how their technology transforms industries
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language
Let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun at weekly in-person and virtual meetings! Here is a photo from our last open house and chartering ceremony:
For more info, refer to the following:
- Flyers: Find Your Voice and Open House
- Website: https://biopacific.toastmastersclubs.org
- Email: contact-6480331@toastmastersclubs.org
PHOTO & VIDEO DISCLAIMER
- Portions of this event will be recorded with photos and videos
- Your ticket purchase implies consent to this recording for the publicity of Toastmasters International and its affiliates (e.g,. BioPacific Toastmasters), without financial compensation or editorial control
Sofia University Auditorium (1069 East Meadow Circle, Palo Alto, CA 94303)
Big data for drug target discovery
Date: Feb 3, 2018, Saturday @ 1:00 pm – @ 3:30 pm
Location: Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080
If any company is interested in sponsoring, please contact sponsorship@cabsweb.org.
When register, please enter your affiliation and name on your photo ID which is required at onsite check-in.
Please register online. Online registration is free. Onsite registration is $10.
1:00 - 1:30PM check-in, onsite registration and networking
1:30PM - 3:30PM
Presentations:

Michael J Keiser PhD, Assistant Professor at UCSF
The Keiser lab combines machine learning and chemical biology methods to investigate how small molecules perturb protein networks to achieve their therapeutic effects. Michael Keiser joined the UCSF faculty in the Dept. of Pharmaceutical Chemistry and the Institute for Neurodegenerative Diseases as an Assistant Professor in 2014, with joint appointments in the Dept. of Bioengineering & Therapeutic Sciences and the Institute for Computational Health Sciences. Before this, he co-founded a startup bringing systems pharmacology methods to pharma and the US FDA. During his bioinformatics Ph.D. at UCSF as a NSF Fellow, Michael developed techniques to relate drugs and proteins from the statistical similarity of their ligands, such as the Similarity Ensemble Approach (SEA). He also holds B.Sc., B.A., and M.A. degrees from Stanford University.
Talk title: Integrating uncertainty into drug-target deep learning

Bin Chen, PhD, Assistant Professor at UCSF
Dr. Bin Chen is an assistant professor in the Institute for Computational Health Sciences at University of California, San Francisco. Dr. Chen is also the founding member of DahShu, a non-profit organization to promote research and education in data sciences. Dr. Chen trained as a chemist in college, worked as a software engineer before graduate school, trained as a chem/bioinformatician in graduate school, worked as a computational scientist at Novartis, Pfizer and Merck. He received his PhD in informatics at Indiana University, Bloomington and pursed the postdoctoral training in Dr. Atul Butte’s lab at Stanford University. His lab is supported by the BD2K K01, one R21, one P01, one U24, and one grant from L’Oreal. His work is recently featured in UCSF News, STAT, GEN, GenomeWeb and KCBS. The liver cancer drug discovery work is featured in the UCSF Magazine Winter 2018 and the UCSF Cancer Year in Review: 2017. More info is available on his lab website (http://binchenlab.org/).
Talk title: Big-Data Analysis Points Toward A New Cancer Drug Discovery Method
Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080
If you were a participant in the 2017 CAN program, please come to mingle with mentors and fellow mentees. If you were not a participant, you are also welcome to come and learn about the 2018 CAN program and how you can be involved.
Please register by 5pm PST on Tuesday, Jan 30, 2018 so we could get a correct head count to order food. If you were a participant in the 2017 CAN program, please also fill out this short evaluation form: https://goo.gl/forms/2ElljGy8fWVF1zu33. Your feedback is very important for us to improve the program!
Barrelhouse in Burlingame (305 California Dr, Burlingame, CA 94010)
Novel antibody platforms and applications
Please register online. Online registration is free. Onsite registration is $10.
1:30 - 2:00PM check-in, onsite registration and networking
2:00PM - 4:00PM
Presentations:
1. Yiyuan Yin, PhD, Scientist at NGM Biopharmaceuticals.

Yiyuan Yin has over 10 years of experience in antibody and protein engineering. Her expertise covers broad aspects of biologics discovery, and has published numerous works on bispecific antibodies, computational designs, antibody display technologies etc. Yiyuan is currently a Scientist at NGM Biopharmaceuticals, where she has spearheaded many technology development programs, and has advanced multiple pipeline projects with her state-of-the-art skills. Prior to that, she did her postdoc research at Genentech with Dr. Paul Carter, who is a renowned pioneer in the field of antibody engineering. Yiyuan received her B.S. from Fudan University in Shanghai, China. During her PhD training at the University of Maryland, she conducted researches in structural biology, biophysics and protein engineering with a focus on immune receptors.
Talk title: Novel antibody platforms and their therapeutic potentials
2. Jianlong Luo, PhD, Associate Professor at UCSF

As a faculty member (Associate Professor) at UCSF, Dr. Jianlong Lou was systematically trained in China, Italy and USA in the life science and medical related fields. As principal investigator or co-investigator or key personal in the research teams or supporting technical expert together with Dr. Andrew Bradbury and Dr. James D Marks, Dr. Lou has been doing antibody engineering over three decades, engaged in at least 35 research grants with total funding over $100 million from different governmental agencies or industrial partners in the past 25 years. Dr. Lou has applied animal immunization, hybridoma, phage display, yeast display, mammalian cell antibody production, computer-based antibody humanization and many other modern molecular engineering technologies for antibody optimization. He has authored or co-authored three scientific books and over 50 peer-reviewed research papers, with thousands of worldwide SCI citations. He is also the active reviewer for papers in five international journals in the life or medical science field. Dr. Lou is the co-inventor for 6 granted and 7 pending US and worldwide patents since 2006. Most of the awarded patents were out-licensed to industry partners, and one of the license deal to a big pharmaceutical partner was the largest (dollar amount) license deal UCSF ever signed till 2012 and also ranked in the top 1% of all license deals from any academic institute to industry. Several human antibody drugs from his invention are in different stages of clinical trial in USA.
Talk title: The Beauty of Synergy: A tri-epitope molecular captures the potency of three mAb combination in BoNT Neutralization.
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
2018 CABS Investor Forum
About the Event:
Chinese American BioPharmaceutical Society (CABS) is very pleased to announce that the 2018 CABS Investor Forum will be held on January 10, 2018 (Wednesday), in conjunction with JP Morgan Healthcare Conference 2018 in San Francisco.
China is determined to become a bigger player in the pharmaceutical market. Chinese investment in the US biotech and health sector has seen a dramatic increase recently. This forum, running from 8:00am-12:00pm, will feature two panels joined by China and US-based investors, business development executives, and entrepreneurs. The discussions will focus on the US-Asia cross border healthcare investment trends, opportunities and challenges.
AGENDA
Wednesday, Jan 10, 2018
Morrison & Foerster LLP
425 Market Street, San Francisco, CA 94105-2482
8:00-9:00
Registration and Networking
9:00-9:10
Welcome Remarks by Alex J. Zhang, PhD, MBA, President, CABS
9:10 -10:20
Panel 1: Art of the Deals between Chinese and US Drug Makers
Moderator:
- Janet Xiao, PhD, JD, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Panel speakers:
- Yaning Wang, PhD, SVP, Qilu Pharmaceutials
- Jin Li, PhD, Founder and CEO, Chengdu HitGen
- Michelle Chen, PhD, Former Executive Director, Merck & Co.
- Jingsong Wang, MD,PhD, Founder and CEO, Harbour BioMed
- Vincent Liu, PhD, Senior Advisor, Fosun Pharmaceutical Group
- Carey Chern, JD, General Counsel, SciClone Pharmaceuticals
- Han Ying, PhD, CSO, Biomedicine Section, Sanpower Group
10:20-10:30
Networking Break
10:30-11:30
Panel 2: Cross Border Venture Investment: What are we Looking for
Moderator:
- Jennifer Hu, PhD, Partner, Qiming U.S. Healthcare Fund
Panel speakers:
- Adam Zhao, PhD, MBA, Founding Partner, Anlong Fund
- John Zhu, PhD, Partner, 6 Dimensions Capital
- William Dai, Managing Partner, ShangBay Capital
- Yao Li Ho, Investment Director, LYFE Capital
- Huijun Zhou, PhD, Founder and CEO, iDNA, President of CABS E-Club
11:30-12:00
Networking
Registration is free and required to attend the forum
Morrison & Foerster LLP 425 Market Street, San Francisco, CA 94105-2482

Happy Holidays from CABS!
On behalf of the CABS Executive Council (EC), We are thankful for having your active participation and strong support to CABS. In 2017, we have successfully organized 48 events, including the 2017 BioPacific Conference, when record-breaking 550 attendees from all over the world celebrated innovation and achievements in life sciences.
In 2018, CABS is going to celebrate the 20th year serving the life science community. Our EC team members have a very exciting lineup of events. The upcoming events include:
- · 2018 CABS Investor Forum on Jan 10 (Wed)
- · Chinese New Year Party on Feb 10 (Sat).
- · 2018 BioPacific Conference (20th Annual Meeting of CABS) on June 23 (Sat).
Please see upcoming events for more details).
As 2017 draws to a close, we sincerely hope that you would kindly consider making a donation to CABS. We are a qualified 501(c)(3) non-profit organization and your charitable contribution may be tax deductible. Furthermore, please also consider leveraging matching funds programs if available from your company to "stretch" your personal donation. Thank you so much for your kind generosity and we really appreciate your strong support to CABS!
We wish you a Merry Christmas and a Happy New Year, and we look forward seeing you at our events in 2018!
For more details on making a donation to CABS, click here
Online
Supporting Pillars, Success Story & Funding Opportunities by Zhongshan Municipal Gov’t
Please note: dinner may not be available if the registration is after 10pm on Dec. 14.
Zhongshan Municipal People’s Government will host “Zhongshan Investment Opportunities Promotion Seminar” on Dec 15, 5:00pm-8:00pm, at Junior Ballroom of Marriott Oakland City Center. We cordially invite you and your colleagues to attend this event.
Besides Mayor of Zhongshan and the delegates, the event will also attract other local officials including California State Officials, Alameda County Officials, Mayor of Oakland, city council members and a lot of representatives of biotech/pharma companies.
Zhongshan is the hometown of Dr. Sun Yat-sen, the great forerunner of China’s democratic revolution. In 2016, the city’s per capita GDP reached $15,000; the total size of R&D investment topped USD ~$1 billion with 7,597 filed patent applications, accounting for 2.4% of the total GDP. In addition, there are 882 high-tech enterprises and 42 post-Ph.D work stations. It also nested over 160 universities and research institutions. The National Health Technology Park in Zhongshan is one of the first national health industrial park in China, which had generated about $6 Billion USD output in 2016. Besides Novartis Group, Ferring, A&Z and other globally well-known pharmaceutical companies, it is also the home for hundreds of biotech/pharma and medical devices companies.
The success of Akeso Bio Pharma is considered a natural outcome from the park incubator program. The company focuses on discovering and developing innovative biologics with international intellectual rights for the treatment of a broad spectrum of diseases, especially in co-developing antibody drugs with strategic partners. Following its A-round seeding investment in July 2015, it soon secured the collaboration with Merck for R&D and commercialization of an immune checkpoint blocking antibody AK107, which lead to as much as $200M USD milestone payment.
We sincerely invite you to attend this limited-seat event, where you could have opportunity to meet and talk with the government officials as well as other associates. The tentative agenda is listed below for your reference.
- · 17:00 – 17:30 Registration
- · 17:30 – 17:50 Keynote speech
- · 17:50 – 18:10 Presentation on Zhongshan investment opportunities
- · 18:10 – 18:30 Success story sharing
- · 18:30 Dinner & Networking
中山市人民政府将于2017年12月15日 在美国加州奥克兰市万豪酒店举行的“中山市投资环境推介会”, 诚挚邀请各位嘉宾出席,会后晚餐和自由交流。
出席此次活动的主要人员有中山市市长及随行代表团、加州州立政府官员、Alameda县政府官员、奥克兰市长、市议员及生物制药领域其他同行们。
中山市是中国民主革命的伟大先行者孙中山的故乡。2016年全市人均国民生产总值达到$15,000 元,研发投入总额达$10亿美元,申请专利7597项,占全市国内生产总值的2.4%。另外市内还有882家高新技术企业,42家博士后工作站,160多所大学和研究机构。中山市国家健康科技园是第一批国家级健康产业园,2016年全年产值约为60亿美元。除了诺华集团、辉凌制药、安士制药等全球知名制药企业,也有数百家的生物/制药和医疗设备公司入住 。
康方生物是一家总部设立在广东省中山市火炬开发区国家健康产业基地的创新型生物制药公司,也是从园区孵化器项目中走出的一个成功案例。公司成立于2012年3月。在2015年7月成功完成A轮融资后,于2015年11月,与默沙东就一项肿瘤免疫疗法的单克隆抗体药物AK107的研究、开发和推广达成合作,并得到以总价为2亿美元的里程碑形式的款项。AK-107是由康方生物在中国发现、用于肿瘤免疫治疗的免疫检查点阻断抗体。康方生物将分享他们在中山市发展的成功经验。
我们诚挚邀请您注册参加此次活动。名额有限,报名从速!下面是此次活动议程供您参考。
- · 17:00 – 17:30 注册
- · 17:30 – 17:50 主要领导发言
- · 17:50 – 18:10 中山投资环境介绍
- · 18:10 – 18:30 康方生物公司介绍成功经验
- · 18:30 – 晚餐及交流
Junior Ballroom on 2nd Floor at Marriott Oakland City Center, 1001 Broadway, Oakland, CA

Want to elevate your professional development to the next level? If so, learn how BioPacific Toastmasters can improve your leadership and communication skills with the following special presentations on mastery:
Become an Effective Communicator - Set Your Mind
- KC Huang, the club mentor, is a long time Toastmaster, member of the Early Risers Club, a club officer since 2003, and a past Division Director of District 4. His educational background is in Electrophysics and Engineering with four decades of experience in the Information Technology service industry. He is also certified in Project Management and Business Analysis.
- This is one of a series of talks based on a speaker's experience and best practices. The purpose of this series is to provide tips and techniques for those who want to enhance their own communications skills.
Inspire Your Audience - Master the Art of Evaluation
- Jenny Liu, the club's VP of Public Relations, applies her evaluation skills as a consultant and an MIT Alumni Angels Officer. As a Toastmaster, she won 2nd Place in the Area B4 Humorous Speech Contest. After graduating from MIT and Hult International Business School, her career path led her from research to marketing at organizations, ranging from startups to Fortune 500 companies, in the Life Sciences. For over a decade, she has been empowering executives to digitally transform Health Care.
- She will guide us on the Roadmap to Success, a method developed by Kristian Crump, a three-time District Evaluation Champion.
Let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun!

Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Commercial Strategy Workshop: Market Access and Reimbursement
Saturday December 2nd, 2017 @ 1:00 pm - 3:00 pm
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Dear CABS members,
The time has (long overdue) come for the next Commercial Strategy Workshop session. The mission of these series of workshops is to provide an overview of the business decision making process in the commercializing of new therapeutics and medical devices. These workshops will be valuable to anyone interested in learning more about the business side of life sciences and potential career paths.
This workshop will cover US market access and reimbursement, as well as the current insurance landscape. The objective of this workshop is to provide a high level overview of how insurance dynamics affect commercial decisions across the entire bio-pharma life cycle (pre-clinical, development, launch, marketing).
Sincerely yours,
Anthony Hsiao
Cost: free for CABS members; $10 for non-members
Registration: online only (space is limited)
![]()
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010

Biopharma Development & Production Asia Pacific 2017
6-9 November 2017
Hilton, Singapore
www.biopharmaproduction-asia.com
Asia’s most influential conference Biopharma Development & Production Asia Pacific continues to deliver its promise. Packed with case studies and refreshed agendas, it will bring together a group of leading minds to address industry’s next steps, market opportunities, disrupted technologies, meeting of regulatory expectations and commercial models that will shape the biopharmaceutical industry in Asia Pacific.
Conference Highlights:
- · 2 Co Located Conferences
- · Bioprocess and Technology - Manufacturing Trends, Capital Spending, Quality & Risk, Future Facilities
- · Biosimilars Markets - Regional Standards and Approvals, Risks, Opportunities and Competitive Advantage
- · 50+ Case Studies, Project Showcases, Commercial and Technical Papers
- · Regulatory Outlook - Interpretation, Guidelines for Biologics, Harmonisation, Disease Surveillance
- · Commercial Insights - Gaining Critical Mass in the Region and Getting the Strategy Right
Exclusive for CABS members: Quote P46346CABS for 20% off normal conference rates!
Need help? Contact our Customer Service & Booking Hotlines
Email: register@ibcasia.com.sg
Telephone: +65 6508 2401
Fax: +65 6508 2407
Hilton, Singapore
About the Topic:
Do you know that more than 30 of the world's leading biopharmaceutical companies are leveraging Singapore as a key home base to drive innovation, commercialization and market growth in Asia? Do you know that the vibrant biopharmaceutical market in Singapore is propelled not only by innovation but also it's pro-business environment, low labor costs and corporate tax rate, and strong government support? Come find out more about the business and tax landscape in Singapore for the biotechnology and pharmaceutical industry, hosted by one of the BIG FOUR business accounting firms PwC.
The workshop will look at Singapore's business environment and touch on its pharma and biotech scene.
We will also talk about the following:
- Possible business structures;
- Entry options;
- Tax environment as well as tax incentives and grants available.
Speakers:
Abhijit Ghosh
Partner, Tax Market Leader and Pharmaceutical & Life Sciences Leader
PwC Singapore
Abhijit is a an International Tax Partner and Pharmaceutical & Healthcare practice leader in Corporate Tax Advisory Services Group of PwC Singapore. Abhijit has more than 27 years of experience in advising and managing various cross-border complex business structures and operational models for MNCs and SMEs as they expand globally.
LENG Harn Szuan
Director, Corporate Tax and Stamp Duty
PwC Singapore
Harn Szuan is a director with PwC Singapore’s Corporate Tax Advisory team and has more than 14 years of corporate and international tax experience. In 2015, Harn Szuan spent the year at the Los Angeles office of PwC US under a joint programme between PwC and a Singapore government economic agency where she focused on developing opportunities with companies keen to invest in Singapore.
Hanqi Investment Inc. 1633 Bayshore Hwy, Burlingame, CA 94010
2017 AI + Pharmaceutical and Healthcare Innovation Summit
(2017人工智能+医药健康创新峰会)
(Call for Startup Application, travel expense supported. See below for details.)
Date:Oct 17-18th, 2017
Location: Shenzhen, Guangdong, China
Organizers:
XtalPi Inc.,
Pfizer Inc.,
Tencent Holdings Ltd.,
Shenzhen Futian District Government,
Peking University Shenzhen Graduate School
Supporting Partners:
CABS
Speakers:
Prof. Kaitai Yao
Chinese Science Academy Fellow
Barbara Sosnowski
Vice President, External R&D Innovation at Pfizer
Dr. Hannes Smarason
CEO, WuXi NextCODE
Dr. Megan Robinson
Director, R&D Strategy and Portfolio, Pfizer
Dr. Nick Clarke
Executive Director, External Science & Innovation in UK, Pfizer
Dr. Liming Shao
Professor, School of Medicine, Fudan University
Ye Yin*
CEO/President, BGI Genomic
Ruiying Li
Cofounder, Chief Scientist, iCarbonX
Dr. Lipeng Lai
Co-Founder, Lead of Big Data and AI R&D, XtalPi
Dr. Wei Chen
Chengdu NetVation-Hitgen CEO
More speakers see brochure in details.
VC Lists:
Tencent Investment
Google Ventures
Sequoia Capital China
ZhenFund
FreesFund
China Life Investment Holding Company Limited
FOSUN TONGHAO
DHVC
Northern Light Venture Capital
Morningside Venture Captical
Genesis Capital
China Resources Szitic Trust Co.
Call for Startup Application:
We are looking for early-stage startups ( after proof-of-concept and before Series B round funding) at the cross-section between big data, AI, and pharmaceutical or healthcare biotech.
The selected team will get travel support and more:
- - A trip to Shenzhen, China, with up to $1,000 travel expense support! Hotel and meals covered.
- - Admission to the Artificial Intelligence + Pharmaceutical and Healthcare Innovation Summit (AIPHIS)
- - Gain access to local resources, key industry contacts, and market insights, all of which could help bring your startup into reality
- - Pitch your startup idea to influential investors who focus in the pharmaceutical and healthcare market from Sequoia, Google, Tencent, ZhenFund, FreesFund, IDG, and NetVation, just to name a few
- - Media exposure in some of the most read Chinese tech/biotech outlets
- - Every team will get $7K credit for Qcloud. The top three will get $15K credit for Qcloud and extra service from our partners that worth $100K.
Send your short BP deck to yanqi.zhang@xtalpi.com before October 2nd where a panel from the organizers will review your startup idea on innovative technology, impact, market potential, team.
About the Event:
Artificial intelligence (AI) is considered the next major technology revolution faced by human society, and many believe it will be the driving force of the 4th industrial revolution. Last month, the Chinese State Council released a guideline on AI development, articulating AI’s strategic importance in the country’s development agenda, and announcing China’s plan to become a “premier global AI innovation center” with dedicated investments to support a prosperous domestic AI economy. On the global level, AI is transforming business and pushing the limit of efficiency and human capacity in many industries, among which life science and biopharmaceuticals are the hottest areas of pursuit for innovators and investors. China, with its huge population and healthcare market, and a fast-growing appetite and ecosystem for AI development, is facing unique opportunities and challenges. In such a context, we are hosting the 2017 Artificial Intelligence + Pharmaceutical and Healthcare Innovations Summit (AIPHIS) in Shenzhen, the “Silicon Valley” of China. The summit features leading pharmaceutical companies such as Pfizer Inc. and Ariad Pharmaceuticals, and up-and-rising AI or big-data-driven biotechnology companies such as XtalPi, and the Tencent AI Research Center. Confirmed speakers also include algorithm scientists from Google, acclaimed researchers and scientists, senior investors in the AI + life sciences sector, and a list of early-stage startups from Boston and the Bay Area who will be presenting some of the most cutting-edge technologies and innovative ideas from the industry and the academia. By creating a conversation platform for the top global players across the sectors, AIPHIS aims to explore the potentials of AI in healthcare and drug research and development, share valuable knowledge and success experiences, stimulate in-depth discussions on AI’s practical implication for the industry, foster a community of trailblazers, and inspire cross-disciplinary collaborations.
See event brochure for detailed agenda. https://drive.google.com/open?id=0B06Vz6jEW2XlU0R3aWhlMmV3S0E
Shenzhen, Guangdong, China
2017 Chinese Bioscience Association Annual Conference
Date & Time: Oct. 14, 2017 (8:00am-4:30pm PDT)
Location: Crowne Plaza, Foster City, CA
Theme: Promoting Biosciences for Better Human Health- Celebrating CBA 20th Anniversary
Complimentary coffee and tea, continental breakfast. Free parking.
Registration:
CBA Members:
$30 online. Lunch included.
$40 onsite. Lunch not guaranteed.
Non-Members:
$50 online. Lunch included.
$60 onsite. Lunch not guaranteed.
Website: http://www.cbasf.org/
Agenda:
|
8:00-8:30 am |
Registration, Breakfast and Exhibitions |
|
8:30 – 8:35 am |
Welcome and Opening Remarks Patrick Yang, Ph.D., CBA president |
|
8:35 – 8:40 am |
Conference Introduction Huifang Li, Ph.D., CBA Conference Chair |
|
Introduction: Lin Sun-Hoffman, Ph.D. |
|
|
8:40 – 9:20 am |
Keynote Presentation: Innovative Drug Development: Maximizing Success and Value Peony Yu, M.D. (CMO, Fibrogen Inc.) |
|
Introduction: Mr. Caesar Ho |
|
|
9:20 – 9:45 am |
Cepheid: from Rags to Riches Kurt Petersen, Ph.D. (Co-Founder, Cepheid Inc.) |
|
Panel Discussion I Moderator: Mr. Caesar Ho & Mr. Ravi Mistry |
|
|
9:45 – 10:25 am |
Entrepreneurship Paul Hung, Ph.D. (CEO, COMBiNATi) Ben Hwang (CEO, Profusa) Dan Laser Ph.D. (CEO, Applaud Medical, Inc.) Ben Wang, Ph.D. (Co-founder, Chimera Bioengineering) Chandler Robinson, M.D. (CEO, Monopar Therapeutics) |
|
10:25-10:40 am |
Networking and Exhibitions |
|
10:40 – 11:05 am |
Build Scientist Town to Save Lives with Frontier Science and Technology Mr. William Wei (VP of America Frontier Science Institute & Chief Architect of American Chinese Friendship Council) |
|
11:05 – 11:30 am |
Opportunities for Biosimilar Companies under U.S. Legal System Patricia Campbell, Ph.D. (Partner, Jones Day) Bing Liang, Ph.D. (Associate Attorney, Jones Day) |
|
11:30-11:45 am |
Sponsors speech |
|
11:45-11:50 am |
Ho Family Scholarship Mr. Caesar Ho |
|
11:50 – 12:00 pm |
CBA 20 years’ Review Patrick Yang, Ph.D. |
|
12:00-12:30 pm |
Lunch and Exhibitions |
|
Introduction: Michelle Chen, Ph.D. |
|
|
12:30 – 1:10 pm |
Keynote presentation: Avastin’s Development: The Case Study for Mega Brand Philippe Bishop, M.D. (CMO, Aratinga.bio, Inc.) |
|
1:10 – 1:35 pm |
Merck San Francisco: Commitment to Science-driven Transformational Medicines James Mu, Ph.D. (Merck Research Laboratories San Francisco) |
|
1:35 – 2:00 pm |
Transformation of BeiGene via Business Development and Partnership Ji Li, Ph.D. (EVP, BeiGene) |
|
2:00 – 2:15 pm |
Networking and Exhibitions |
|
Panel Discussion II Moderator: Michelle Chen, Ph.D. |
|
|
2:15 - 2:55 pm |
Biotech Investment Jennifer Hu, Ph.D. (Partner, Qiming US Healthcare Fund) Alexi Ji, Ph.D./MBA (Partner, Illumina Ventures) Rajeev Dadoo, Ph.D./MBA (Partner, SR One) Vincent Xiang, Ph.D./MBA (Partner, Six Dimensions Capital) |
|
Panel Discussion III Moderator: Patty Kiang, Ph.D. |
|
|
2:55 – 3:45 pm |
Biotech Supply Chain Lee-Cheng Liu, Ph.D. (President & CEO, Eirgenix) Ray Chen, Ph.D. (Sr. Director, Genscript) Amit Chaudhuri, Ph.D. (VP, Medgenome) Dan Zhang, Ph.D. (CEO, Fountain Medical Development) |
|
Panel Discussion IV Moderator: Lin Sun-Hoffman, Ph.D. |
|
|
3:45 – 4:15 pm |
East West Convergence - One Mind, Two Coasts Patrick Yang, Ph.D. (CBA president, west coast) Dong Shen, Ph.D. (CBA president, east coast) |
|
4:15 – 4:25 pm |
Former CBA President Speech Shian-Jiun Shih, Ph.D. (CBA founding President) |
|
4:25 – 4:30 pm |
Closing Remarks Huifang Li, Ph.D., CBA Conference Chair |
|
4:30 – 7:00 pm |
Networking Reception |
Crowne Plaza, Foster City, CA
CABS Executive Council (2017-2018) Election Application is Now Open!
Dear CABS members and friends,
CABS has been proudly serving 3000+ members, sponsors and friends of the life sciences communities in the U.S. Bay Area, China and Pacific Rim countries for the last 19 years. We organize year-round programs and workshops to showcase the latest advancements in life sciences, foster collaborations and entrepreneurship, facilitate mentorship and career development and create networking opportunities for our members.
We are now calling for candidates to run for our Executive Council (EC) for 2017-2018.
Our EC is the heart and soul of CABS, and orchestrates our activities throughout the year. In October, the new EC will be taking the leadership role in planning and organizing exciting new programs and initiatives both locally and abroad. This is your opportunity to lead and to make significant contributions to our organization. With your participation, we can elevate CABS to the next level!
To participate in our election, please kindly submit your application here no later than October 7, 2017. We will have a separate announcement regarding the voting process.
Very much look forward to working together and really appreciate your kind support !
Alex J. Zhang, PhD
President-Elect, CABS
2017 SVIEF
US China MedTech Innovation Summit
(2017硅谷高创会智慧医疗峰会)
Date:September 29, 2017 @2:45pm-5:00pm
Location: Santa Clara Convention Center
Speakers: Rafael Gomez-Sjoberg(Chan Zuckerberg Biohub);David Rhew,(Samsung SDS);Huijun Zhou(iDNA);Walter Stewart(Sutter Health)
Organizers: SVIEF
Date: 9/29/2017
Time: 2:45-5:00pm
Registration Fee: $80 Afternoon Pass (CABS Members get 50% off using code iDNA50)
Location: Santa Clara Convention Center
Registration Link: here
About the Topic:
Silicon Valley Innovation & Entrepreneurship Forum (SVIEF) is the largest cross-border high-tech forum held in the Bay Area. For consecutive 7 years, we have successfully organized international mega summits in both China and the U.S. Our goal is to foster learning and to provoke conversation and collaboration that matter
The 2017 SVIEF hosts the US China MedTech Innovation Summit as a way to bring together all stakeholders in healthcare to build the relationships and ignite the technologies that will advance patient care. Join us as we highlight the technologies, discuss the opportunities, and navigate the future challenges of this exciting new market.
About the speakers:
David Rhew, Samsung SDS(CMO and Vice-President for Global Healthcare)
Prashant Natarajan,oracle (Director of Product Strategy)
Rafael Gomez-Sjoberg,Chan Zuckerberg Biohub(Director of Bioengineering)
Huijun Zhou, iDNA(founder/CEO)
John Adler,Cureus Inc (CEO);
Xiang Qian,Stanford Medicine X Stanford Hospital (Director of International Medical Services Advisor)
Walter Stewart, Sutter Health(Chief Research Officer)
Hongye Sun , WuXi NextCODE (CTO)
Oscar Edward Streeter, California Division of the American Cancer Society (Chief Medical &Scientific Officer and Board Member)
Charlene Yu Vaughn, Algonquin Group(CEO/ Founder)
More speakers info: http://www.svief.org/2017/sv/
Webcast: Conducting Clinical Trials in China – Regulatory and Clinical Challenges and Recent Developments for Drug and Device Development; Lessons for US-based Companies Entering the China Market
Speakers: Lan Huang (BeyondSpring); John Mao (Foresee Pharma); Vivian Mao (Abbott Vascular); Honggang Bi (Covance China); Guobin Wang (PPD China)
Organizers: PBSS-CABS
Date: 9/21/2017
Time: 14:00-16:30
Registration fee (USD): $25 (CABS Members get $10 off using code 1170921cabs)
Registration Link: here
Location: Online Webcast ONLY
Registration deadline:9/20/2017 (it will close sooner if the seating cap is reached)
About the Topic
Pharmaceutical and Medical Device companies today face a growing demand to expand their clinical trial programs into China in order to access the vast pool of patients. While there are clear benefits for conducting clinical trials in China, there are significant operational and regulatory challenges, which need to be addressed before overseas companies plan to conduct trials in China, either as a trial conducted solely in China or as part of a multinational trial involving Chinese patients. It is important that appropriate measures are put in place to satisfy the ever evolving Chinese clinical trial and approvability standards. In the meantime, the China FDA (CFA) has initiated a major effort to harmonize the regulatory and clinical practices with the developed world and has implemented a number of policies that have profound impact on the clinical and regulatory practices in China.
This workshop aims at discussing some of the regulatory and operational challenges when conducting clinical trials in China, in both, the Pharma and Medical device arena. The presenters who are industry leaders with deep experience with drug and device development in China will review some of the recent regulations for the Pharma and Device development in China and suggest ways to approach China’s FDA (CFDA). It will discuss challenges and solutions when conducting national or multinational device trials in China from the perspectives of a local Chinese CRO as well as global CROs with affiliates in China. The workshop will provide examples for early strategies to include China in the development as part of a global development plan. A case example will demonstrate how a global clinical Phase 3 trial would suffice for a US FDA and China FDA approval.
About the Speakers
Lan Huang, PhD, BeyondSpring (Co-founder & CEO)
John Mao, PhD, Foresee Pharma (SVP)
Vivian Mao, MD, Abbott Vascular (Associate Director, Clinical Science)
Honggang Bi, PhD, Covance China (Corporate Vice President and General Manager)
Guobin Wang, PhD, PPD China (Director, Project Management)
Online Webcast ONLY
CABS E-Club September 2017 Event
Fireside Chat about the Current State and Future Trend of Digital Health in China
Speakers:
David Chen, Co-Founder, former Vice Chairman & President of VanceInfo; Chairman of HYSTA (Hua Yuan Science and Technology Association); Operating Partner, Sequoia CBC Cross Border Digital Fund; Venture Advisor of West Summit Capital; Chief Advisor of CEC Data
An Doan, Attorney, Jones Day
Location: Jones Day office in Palo Alto, 1755 Embarcadero Rd, Palo Alto, CA 94303
Time: September 16, 2017, Saturday, 1:30-4:00pm
Agenda:
1:30-2:00 Registration and networking
2:00-3:30 Fireside chat and round-table discussions
3:30-4:00 Networking (refreshments provided)
Registration: here
Dear E-Club members,
I am excited to announce that we are honored to have Mr. David Chen, a successful founder, investor, and industry veteran in the IT sector, and Ms. An Doan, an attorney at Jones Day, to give us a fireside chat about the digital health industry.
Mr. David Chen will give us a picture of the current state of the digital health industry in the US and China and share his wisdom on the future trends of digital health in China based on his long-time experience operating companies, consulting VC firms, and interacting with regulatory agencies in China. Ms. An Doan has kindly agreed to moderate the chat and share her understanding about digital health based on her experience representing technology companies as an attorney.
This is a great opportunity for people who are interested in starting, running, or investing in digital health companies to get fresh perspectives about this industry. Come to spend time and share insights with fellow E-Club members, partners and collaborators.
Please register here by Friday, September 15th if you would like to join the event. Look forward to seeing many of you on September 16!
Best regards,
Alexis Ji
Chair, CABS E-Club
Speakers

David Chen
Co-Founder, former Vice Chairman & President of VanceInfo
Chairman of HYSTA (Hua Yuan Science and Technology Association)
Operating Partner, Sequoia CBC Cross Border Digital Fund
Venture Advisor of West Summit Capital
Chief Advisor of CEC Data
David Chen is co-Founder and former Vice Chairman & President of VanceInfo, one of the largest China based IT services firms with more than 20,000 associates. He oversaw strategy and daily operations between 2001 and 2010. Before it went public in 2007 at NYSE, VanceInfo was backed by prominent VCs including DCM, Sequoia Capital and Legend Capital.
Prior to co-founding VanceInfo, David held multiple management and technical positions at Asera, IBM/Crossworlds, KPMG Consulting, Oracle and UCSF Medical Center.
David is passionate about fostering the spirit of entrepreneurship and technological innovation across China and the US. He is the Chairman of HYSTA, a nonprofit organization dedicated to entrepreneurship and leadership among Chinese professionals and entrepreneurs in Silicon Valley. He is also an active angel investor and currently serves as board member for sense.ly, Medicinovo and SocialTouch.
David earned his degree of Master of Science in Computer Engineering from University of California, Irvine and Bachelor of Science in Electrical Engineering from Tongji University.

An P. Doan
Attorney, Jones Day
650-739-3913, apdoan@jonesday.com
Ms. An Doan practices intellectual property law with emphasis on technology transactions involving the development and transfer of intellectual property rights. She advises clients on structuring, drafting, and negotiating IP development agreements (e.g., strategic alliance, joint venture, joint development, collaboration, and research agreements); IP commercialization and license agreements; and services, supply, manufacturing, OEM, reseller, and distribution arrangements, among other complex technology transactions. An also represents buyers and sellers in domestic and cross-border corporate transactions such as mergers and acquisitions, counseling on intellectual property matters including due diligence and transitional arrangements. She has extensive experience in the computer hardware and software, education, and energy industries.
An also represents clients in patent litigations in federal courts and in post-grant proceedings before the USPTO. She has represented companies in patent cases relating to a variety of technologies, including semiconductor circuitry, fabrication, and packaging, GPS, display systems, image and video compression, signal processing, and smart cards. An has significant experience handling all stages of patent litigation, including developing and executing case strategies, managing discovery, preparing infringement and invalidity contentions, taking and defending depositions and preparing witnesses, drafting expert reports and dispositive motions, arguing motions, and preparing post-trial briefs.
In 2016, An spent most of the year at a firm client, Chevron Corporation, providing legal support to Chevron Technology Marketing (TEMA) — the commercialization center for Chevron's hydrocracking and refinery technology. In her capacity as TEMA's corporate counsel, An handled all legal matters for TEMA, including advising the business team on structuring commercial relationships, preparing and negotiating commercial and technology contracts, and counseling on corporate governance issues.
Jones Day office in Palo Alto, 1755 Embarcadero Rd, Palo Alto, CA 94303

Visit us during the BioPacific Toastmasters Open House and celebrate our Club Chartering ceremony with a keynote speech from our District 4 leader and refreshments. To elevate your thinking (with "brain lifting"), leadership, and communication skills to the next level, here are some ways how Toastmasters International (TI) can help develop your personal life and professional career:
- Scientists can break the ice and enrich presentations by researching their topic and organizing their speech with visual aids that inspire their audience
- Entrepreneurs can practice getting to the point or telling stories when pitching how their technology transforms industries
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language
Thanks to sponsorship by the Chinese American Biopharmaceutical Society (CABS) for chartering a Toastmasters club, let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun! Here is a photo from our first contest where participants received a FREE code (worth $200) for a presentation and public speaking course by Chris Haroun, an award-winning professor, author, and venture capitalist.

For more info, refer to the following:
- Fliers: Find Your Voice and Open House
- Schedule:
-
- Open House (10 AM - 11 AM)
- Club Charter Ceremony (11 AM - 11:30 AM)
- Celebration (11:30 AM - 12 PM)
- Website: https://biopacific.toastmastersclubs.org
- Contact: Ting Ye, the BioPacific Toastmasters President (tm[dot]cabs[at]cabsweb[dot]org)

Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
The event will be in Chinese. No need to register.
Event details. Simcere Stanford Lecture.pdf
.jpg)
Li Ka Shing Learning and Knowledge Center, Room LK120
CABS 2017 Summer BBQ
Date: August 26th, Saturday, 11 am-3 pm
Location: Boothbay Park, Foster City
You and your family are cordially invited to our Annual Summer Networking Picnics, hosted by CABS Social Life Committee.
BBQ grilled pork, beef, chicken, hot dog, hamburger, salad, soft drinks, fruits, desserts and a lot more!
All you can eat and drink! With a bonus of unlimited FUN and unexpected opportunity for making new friends and social/career interactions!!
Lucky draws: $25 gift cards and other secret surprising!!!
Online registration is strongly recommended.
Registration Fee.
Online:
Active CABS member: $5
Non-member: $10
Senior >65 or Child <15: $1
Onsite:
Active CABS member: $10
Non-member: $15
Senior >65 or Child <15: $1
On-line Registration closes on August 25th, 12:00 PM (noon).
Boothbay Park, Foster City

Practically AI - Discover How AI is Powering Technology Innovation
Event Date: August 24th (Thursday) - 6:30pm to 9:15pm
Location: Visa Inc - 900 Metro Center Blvd, Foster City, CA
Registration: https://www.meetup.com/practically-ai/events/241947965/
Schedule
6:30pm - 7:00pm – Check In, Food, Networking
7:00pm – 9:00pm – Presentation, Q&A
9:00pm - 9:15pm – Closing
We are honored to have Visa host the Practically AI Meetup where some of the brightest minds in artificial intelligence gather in Foster City to share how they implement AI in real world projects. Join us as we explore the future of AI and how to get there from here. Each Speaker will have the opportunity to present their areas of knowledge and expertise regarding applications of Artificial & Augmented Intelligence, Machine Learning, Deep Learning, Computer Vision & Expert Systems depending on their subject focus. You will get exposure to differnet methods experts, entrepreneurs and companies are using AI today.
CABS members will get a 30% discount for the event. The discount code on Eventbrite is “CABS”
Visa Inc - 900 Metro Center Blvd, Foster City, CA
Updated information: due to a schedule conflict, this event will be postponed to August. 19 (Saturday) at 6:30-9pm. Chairman will not be able to make it but Dr. Sun (global research VP) will still give the speech, and meet people with projects and looking for a job.
High level officer from Tasly Group (top 10 pharmaceutical companies in China) will be in the bay area. Tasly is looking for project collaboration and talent recruitment opportunities (details below). In addition, there will be a seminar on FDA/CFDA regulatory affairs.
If you would like to present for 5min in the areas that Tasly is interested, please fill out the google form below. We will get back to you. https://docs.google.com/forms/d/e/1FAIpQLSdAPRhwIThhYwewc0gxf73HjIssy5YgKnuDFxy6R5KbT3jMJA/viewform
If you apply for the job positions, please bring your resume and attend the meeting.
If you just want to attend the seminar, we welcome you as well.
Please register if you plan to show up no matter to present, to apply for job, or to attend the seminar.
If you have any questions, please email at fundraising@cabsweb.org
Agenda
6-6:30pm networking
7-8pm presentation: Active Adaptation and Fusion of Regulatory: Challenges and Opportunities for Pharmaceutical Companies in China and the U.S. by former FDA high-level officer and current global research VP of Tasly Group
8-9pm project presentation (5min each)
Tasly Group Introduction
Tasly Holding Group was founded in 1994, a high-tech and globalized enterprise focused on Great Health industry, with pharmaceutical industry as its core business; health care industry, health management and service industry as its paralleling businesses. The corporate philosophy of Tasly is ‘To pursue harmonization between human and nature, to improve life quality’; the corporate mission is ‘To share the joy of health with all’. Tasly is committed to build the best modern and international TCM brand, and to become a leading brand in Great Health industry. Tasly’s total capital has reached 49.2 billion RMB and the sales volume is 30.4 billion RMB in 2016.
Tasly has developed a series of products, including modern TCM, chemical medicines, as well as biological drugs. The signature product Compound Danshen Dripping Pills has successfully completed FDA Phase III clinical trials in 2016; and it is the first compound Chinese herbal drug which have completed FDA-regulated phase-III clinical trials and proved that it is safe and effective in the treatment of chronic stable angina. Series of Tasly products are currently in the pipeline under globalization development.
Dr. Henry H. Sun Introduction
Dr. Sun received his PhD in Clinical Pharmacology and Biopharmaceutics from the University of Connecticut in 1993.He served at the US FDA as a regulatory reviewer, then was promoted to one of the top-ranked agency-level Expert Scientist/Reviewer in 2000. In 2006,Dr. Sun joined Tasly Group Co. Ltd. as Vice President. Dr. Sun is currently global research VP of Tasly, President of Tasly Pharmaceuticals Inc., and founder and CEO of Aexon Research, Inc., a full service global CRO firm focusing on regulatory consultations and clinical research programs. Dr. Sun also serves at Tianjin University as a Professor and Department Head of Regulatory Science and Clinical Pharmacology, and is a Special Professor at the School of Medicine, University of Nottingham of UK, Adjunct Research Scientist at the NIH of USA.
Interested Projects/Targets
- Disease areas:cardiovascular diseases, cancer, hepatobiliary & digestive diseases, gynecological & pediatric diseases, neurological diseases
- Type: innovative new drugs (NMEs, biosimilar), innovative diagnostic techniques, innovative technology platforms (drug delivery, analytical method, research model, etc.), novel therapeutic program/scheme, small to medium biotech companies (investment, M&A), research & marketing collaboration
Talents Recruitment
- Management: senior executive, senior BD, senior marketing & sales (in Washington DC area or in China including Tianjin, Shanghai Zhangjiang, and Jiangsu Hauian)
- Technical experts: chemical and biological drug research (in Tianjin, Shanghai Zhangjiang or in Jiangsu Huaian), global clinical research, and others who specialized in certain drug research areas (in Washington DC area)
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010

Are you interested in learning how Toastmasters International can help improve your leadership and communication skills? If so, attend the Toastmasters Leadership Institute event to:
- Gain the professional edge you seek to succeed and influence decision-making
- Network with Toastmasters members and non-members as professionals
- Receive credit for Toastmasters club officer training
In addition, learn from the following educational sessions:
- Neuroscience of Being Memorable
- Positive Leadership
- Stories Sell
- Effectively Facilitating Discussions & Managing Difficult Behaviors
- Powerpoint: Mastering the Medium
- Humorous Speaking
- Leveraging with LinkedIn
- Applying Toastmasters to Real Life
Register HERE by Friday, August 4th!
----------------------------------------------------------------------------
To check out the Biopacific Toastmasters club, email Ting Ye, the President, at tingye2012[at]gmail.com.
Genentech (350 DNA Way, Building 35, South San Francisco, CA 94080)

Want to elevate your professional development to the next level? If so, here are some ways that Toastmasters International (TI) can improve your thinking, communication, and leadership skills:
- Scientists can break the ice and enrich presentations by researching topics and organizing speeches with visual aids that engage and inspire audiences
- Entrepreneurs can practice getting to the point and telling stories when pitching how their technology transforms industries
- Business professionals can enhance the impact of their talks by persuading audiences with power through word choice, rhetorical devices, vocal variety, and body language
Thanks to sponsorship by the Chinese American Biopharmaceutical Society (CABS) for chartering a Toastmasters club, let's overcome America's #1 fear of public speaking together while talking about various topics, networking, and having fun at meetings in-person and virtually! Here is a photo from our first contest where participants received a FREE code (worth $200) for a presentation and public speaking course by Chris Haroun, an award-winning professor, author, and venture capitalist.

Here are the details for active CABS members to visit us:
- Time: Saturdays from 10 - 11AM (except during holiday weekends)
- Policy: Guests are welcome to attend two meetings for free
- Price: Toastmasters International collects a one-time new membership fee ($20) with CA sales tax ($1.55) and six-month membership dues ($45) for use of their materials and a FREE subscription to Toastmaster magazine
- Club info: #06480331 in Division B, Area 4, and District 4
- Website: https://biopacific.toastmastersclubs.org
- Newsletters: 2018 editions
- Email: contact-6480331@toastmastersclubs.org
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)

Are you interested in elevating your career development by taking it to the next level? If so, here are some examples of how Toastmasters International can help improve your leadership and communication skills:
- Scientists can enrich presentations by organizing the right amount of context, detail, and visual aids for various stakeholders
- Entrepreneurs can practice pitching with storytelling techniques that illustrate how their technology transforms industries
- Business professionals can enhance the impact of their talks by engaging audiences with their powers of persuasion through vocal variety and body language
Thanks to sponsorship by the Chinese American Biopharmaceutical Society (CABS) for chartering the Biopacific Toastmasters Club, let's overcome America's #1 fear of public speaking together among fellow CABS members while talking about various topics, networking, and having fun!
Here are the details for active CABS members to check us out:
- Time: Saturdays from 10 - 11AM
- Location: Hanqi Investments (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
- Materials: agenda and meeting roles sign-up sheet
- Policy: Guests are welcome to attend two meetings for free
- Price: Toastmasters International collects a new membership fee ($20) with CA sales tax ($1.60) and six-month membership dues ($45) for their materials
- Contact: Ting Ye, the BioPacific Toastmasters Club President (tingye2012[at]gmail.com)
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Seminar Luncheon: Developing Relevant Human Neural In Vitro Assays for CNS Safety Assessment and Drug Discovery: Challenges and Perspectives
Organization: PBSS - San Francisco Bay
Date/Time: 7/28/2017; 11:00 AM-1:30 PM
Location: San Francisco Bay Area (Crowne Plaza, Foster City)
Registration Link: http://pbss.org/aspx/eventInfo.aspx?eID=541
San Francisco Bay Area (Crowne Plaza, Foster City)
CABS and San Francisco Healthcare Drinks are Co-hosting a New Event:
Topic: US-CHINA Healthcare Transaction Lessons
Date: July 27th, Thursday from 6:30pm to 9:00pm
On Thursday, July 27th, San Francisco Healthcare Drinks, CABS, and ChinaMed Capital will host a talk by Carey Chern, General Counsel of SciClone entitled 'Lessons Learned from US-CHINA Cross Border Healthcare Transactions'. Chinese life sciences companies and Investors (e.g., PE and venture capital firms) are interested in doing transactions with US companies, including M&A, licensing and other commercial transactions. Carey will provide his legal perspective on US companies entering into transactions with Chinese entities.
About Carey: Carey Chern joined SciClone in October 2016 and serves as General Counsel and Corporate Secretary, as well as SciClone's Chief Compliance Officer. Prior to joining SciClone, from 2011 to 2016, Mr. Chern served in various roles at The Clorox Company covering healthcare matters. Prior to The Clorox Company, Mr. Chern served in various roles at Amgen Inc. Prior to Amgen, Mr. Chern served as an attorney in several large international law firms covering life sciences and corporate transactions matters. Mr. Chern earned a J.D. from Stanford Law School and his A.B. in Economics and Government from Harvard University.
Event Location: 1633 Old Bayshore Hwy, Suite 280, Burlingame, CA 94010.
Contact Us:Brian Hakim: +1(415)-902-6510/ Clare Gu+86-186-163-16925
 RSVP: To RSVP, please send your full name and company name to
RSVP: To RSVP, please send your full name and company name to
Sanfrancisco@healthcaredrinks.com
Hanqi Investment, 1633 Old Bayshore Hwy, Suite 280, Burlingame, CA 94010
CABS E-Club July 2017 Event
Patent Protection and Licensing Strategies for Life Sciences Entrepreneurs
Speakers:
Janet Xiao, Ph.D., J.D., Partner, Morrison & Foerster LLP
Scott Carter, Ph.D., J.D., Of Counsel, Morrison & Foerster LLP
Location: Morrison & Foerster LLP, 755 Page Mill Road, Palo Alto, CA 94304
Time: July 22, 2017, Saturday, 1:30-4:30pm
Agenda:
1:30-2:00 Registration and networking
2:00-4:00 Workshop and round-table discussions
4:00-4:30 Networking (refreshments provided)
Registration: here
Dear E-Club members,
I am excited to announce that we have invited experienced attorneys at Morrison & Foerster, Dr. Janet Xiao and Dr. Scott Carter, to host an IP workshop in the upcoming July event. In this workshop, you will learn about the legal side of founding a life sciences startup, and what it takes to setup your new venture for long-term success. This session will arm you with the knowledge an entrepreneur needs to manage the IP and licensing aspects of launching and growing a startup, and to avoid common mistakes that can have serious consequences down the road. Recent case studies will be presented throughout the program.
Topics covered by this session include:
- What constitutes a strong patent portfolio
- Legal requirements for patentability; recent changes in the legal landscape
- Overview and key considerations of the patent application process
- Key considerations during the IP due diligence process
- In-licenses (universities, research institutions, and research tools)
- Planning for collaborations and out-licenses
This is a great opportunity to learn from experienced attorneys in the Bay Area who counsel innovative companies at all stages of growth. Come to spend time and share insights with fellow E-Club members, partners and collaborators.
Please register here by Friday, July 21th if you would like to join the event. Look forward to seeing many of you on July 22!
Morrison & Foerster LLP, 755 Page Mill Road, Palo Alto, CA 94304

Are you interested in elevating your career development by taking it to the next level? If so, here are some examples of how Toastmasters International can help improve your leadership and communication skills:
- Scientists can enrich presentations by organizing the right amount of context, detail, and visual aids for various stakeholders
- Entrepreneurs can practice pitching with storytelling techniques that illustrate how their technology transforms industries
- Business professionals can enhance the impact of their talks by engaging audiences with their powers of persuasion through vocal variety and body language
Thanks to sponsorship by the Chinese American Biopharmaceutical Society (CABS) for chartering the Biopacific Toastmasters Club, let's overcome America's #1 fear of public speaking together among fellow CABS members while talking about various topics, networking, and having fun!
Here are the details for active CABS members to check us out:
- Time: Saturdays from 10 - 11AM
- Location: Hanqi Investments (1633 Old Bayshore Highway, Suite 280, Burlingame, CA 94010)
- Policy: Guests are welcome to attend two meetings for free
- Price: Toastmasters International collects a new membership fee ($20) with CA sales tax ($1.60) and six-month membership dues ($45) for their materials
- Contact: Ting Ye, the BioPacific Toastmasters Club President (tingye2012[at]gmail.com)
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
Minisymposium: Immuno-Oncology: Advances in Translating from Bench to Clinic
Organization: PBSS - San Francisco Bay
Date/Time: 6/28/2017; 8:30 AM-5:00 PM
Location: San Francisco Bay Area (Crowne Plaza, Foster City)
Registration Link: http://pbss.org/aspx/eventInfo.aspx?eID=534
San Francisco Bay Area (Crowne Plaza, Foster City)
2017 CBA Annual Conference
22nd CBA Annual Conference
Delivering Lifesaving Medicines to Patients through Innovation, Regulatory Reform and Global Partnership
June 25, 2017 | Gaithersburg, Maryland, USA
The Chinese Biopharmaceutical Association (CBA-USA), one of the largest Chinese professional associations in the US, is going to collaborate with MedImmune and co-host its 22rd annual conference on June 25, 2017 at the beautiful campus and the state-of-art conference facility of MedImmune, a member of the AstraZeneca group, the largest biopharma company in the state of Maryland, in Gaithersburg, Maryland. On behalf of the conference organizing committee, we cordially invite Arraybridge to sponsor and exhibit at the event.
The theme of the 22rd CBA annual conference is “Delivering Lifesaving Medicines to Patients through Innovation, Regulatory Reform and Global Partnership”. The theme has been carefully selected to address the critical importance of worldwide collaboration and partnership to capture the great opportunity for the advancement of innovative medicine and biopharmaceuticals in both USA and China.
There will be six sessions:
- Plenary Keynote Presentations and Awards
- Innovative & Precision Medicine
- Regulatory Reform
- Global Partnership
- Project Think Tank & Entrepreneurship
- CBA-SCBA Joint Session
Panel discussions on legal, regulatory aspect and entrepreneurship will also be organized to discuss related issues. The conference provides a perfect venue for people from government, private agencies, biotech and pharmaceutical companies interested in policy issues, business development, and partnership in US and China.
In addition to a compelling conference program, CBA will present its 2017 Brilliant Achievement Award at the Conference, recognizing individuals of exemplary accomplishments in promoting collaboration and global development involving the biopharmaceutical sector in China. We anticipate more than 800 attendees will attend the 2017 Conference.
More meeting details will be announced soon at www.cba-usa.org.

Gaithersburg, Maryland, USA
2017 BioPacific Conference
Theme: Leading the New Wave of Life Sciences Innovation – Technology, Policy, and Investment Trends across the Pacific Rim
Our meeting will showcase:
- Winners of 2017 CABS K. Fong Award in Life Sciences
- Most promising areas in life sciences including cancer immunotherapy, genomics, and big data analytics
- Success stories of entrepreneurs and emerging companies
- Development of Innovative Therapeutics in China
- IP Considerations in Cross-border Deals between China and US Companies
- US-China Cross-Border Healthcare Investment & Entrepreneurship
- BioPartnering Forum to facilitate interactions including project introduction and talent recruitment between attendees and sponsors
Registration:
Breakfast, refreshments, lunch and evening reception are included.
| CABS Member | Early Bird Registration (before 06/10/2017) | Regular Registration (06/11/2017 - 06/24/2017) |
| General | $60 | $100 |
| Student or Postdoc** | $50 Please use your institution email (.edu) |
$90 |
| Non-Member* | Early Bird Registration (before 06/10/2017) | Regular Registration (06/11/2017-06/24/2017) |
| General | $90 | $130 |
| Student or Postdoc** | $80 Please use your institution email (.edu) |
$120 |
* Join CABS today ($30 for one year membership) to save $ on registration fee: http://www.cabsweb.org/members/
** Seeking for volunteers to support our conference. Free conference admission available for selected volunteers.
Email: membership@cabsweb.org
Confirmed Speakers and Panelists include:
- Thomas Hudson, MD, VP & Head of Oncology Discovery & Early Development, AbbVie
- Atul Butte, MD, PhD Director, Institute for Computational Health Sciences, UCSF
- Yinxiang Wang, PhD, Co-Founder, President and CSO, Betta Pharmaceuticals
- Edgar Engleman, MD, Managing Partner, Vivo Capital, and Professor of Pathology and of Medicine, Stanford University
- David Flores, MBA, Founder and CEO, BioCentury
- Xun Xu, MBA, President, BGI Research
- Hua Tu, PhD, CEO, Lakepharma
- Dongliang Ge, PhD, CEO, Apostle, Inc.
- Cheng Liu, PhD, CEO, Eureka Therapeutics
- Alexis Ji, PhD, MBA, Partner, Illumina Ventures
- Tarangini Deshpande, PhD, Founder & CEO, NuMedii
- Michael An, MD, PhD, Founder and CEO, Curegenix
- Wei Zheng, PhD, Founder and CEO, Suzhou Connect
- Baofu Ni, PhD, VP, Kelun Bo-Tai BioPharmaceuticals Co.
- Tao Huang, PhD, Venture Partner, Cenova Capital
- Vincent Xiang, PhD, MBA, Partner, 6 Dimensions Capital
- Karen Liu, PhD, Managing Partner, 3E Bioventures
- Julie Papanek, MBA, Partner, Canaan Partners
- Ashish Xavier, PhD, MBA, VP, Venture Investments, JNJ
- Lu Zhang, MBA, Associate Director of Business Development, Ascentage Pharma
- Johnny Sun, Co-founder and CIO, AccuraGen
- Jie Zhou, PhD, JD, Partner Morrison & Foerster
- Li Feng, PhD, JD, Partner, Finnegan
- Betty H. Chen, JD, Principal, Fish & Richardson
-
More to come
Conference Agenda:
2017 BioPacific Conference (19th Annual Conference of CABS)
Leading the New Wave of Life Sciences Innovation – Technology, Policy, and Investment Trends across the Pacific Rim
|
8:00 AM – 8:30 AM |
REGISTRATION |
Morning Session I: Welcome & Keynote Speeches
Session Chair: Yang Tian, PhD, and Carrie Wang, MD
|
8:30 AM – 8:35 AM |
Welcome Remarks Alex J. Zhang, PhD, MBA, President-Elect of CABS & 2017 BioPacific Conference Organizing Committee Chair |
|
8:35 AM – 8:45 AM |
A Year of Many Firsts at CABS Cheni Kwok, PhD CLP, President of CABS |
|
8:45 AM – 9:20 AM |
Immuno-oncology: What’s next? Thomas Hudson, MD, VP, Head of Oncology Discovery and Early Development, AbbVie |
|
9:20 AM – 9:55 AM |
Translating a Trillion Points of Data into Therapies, Diagnostics, and New Insights into Disease Atul Butte, MD, PhD, Director, Institute for Computational Health Sciences and UCSF Distinguished Professor of Pediatrics, University of California, San Francisco |
|
9:55 AM-10:10 AM |
BREAK |
Morning Session II: 2017 CABS K. Fong Award
Session Chair: Wentao Zhang, PhD
|
10:10 AM-10:20 AM |
CABS K. Fong Award in Life Sciences Presenter: Kenneth Fong, PhD, Chairman, Kenson Ventures |
|
10:20 AM – 10:35 AM |
Award Acceptance Speech (via Video) Yinxiang Wang, PhD, Co-Founder, President, and CSO, Beta Pharmaceuticals |
|
10:35 AM – 11:05 AM |
Immunotherapy for Cancer: A Paradigm Shift Edgar Engleman, MD, Managing Partner, Vivo Capital, and Professor of Pathology and of Medicine, Stanford University |
Morning Session III: Panel Discussion
Session Chair: Yan Wang, PhD, and Gavin Lu, PhD
|
11:05 AM – 11:20 AM |
Diamond Sponsor: Chengdu Medical City Zhao Yang, President of Chengdu Geunion Investment Co., Ltd |
|
11:20 AM – 11:55 AM |
IP Considerations in Cross-border Deals between China and US Companies Moderator, Gini Deshpande, PhD, CEO, NuMedii Panelist, Jie Zhou, PhD, JD, Partner, Morrison & Foerster, LLP Panelist, Li Feng, PhD, JD, Partner, Finnegan Panelist, Betty Chen, JD, Principal, Fish & Richardson |
|
11:55 AM – 12:15 PM |
Platinum Sponsor: Singapore-Sichuan Hi-Tech Innovation Park Wilson Lee, Director, Sino-Singapore (Chengdu) Innovation Park Development Co., Ltd. Platinum Sponsor#2: Kelun Pharmaceuticals Group Co., Ltd. Baofu (Paul ) Ni, PhD, VP, Kelun Bo-Tai Biopharmacetical Co. Ltd., and Senior Director of Kelun Pharmaceutical Research Institute |
| 12:15 PM – 1:00 PM |
LUNCH BREAK |
Afternoon Session I: Keynote Speeches
Session Chair: Wenfeng Xu, PhD, Ziyang Zhong, PhD, and Naibo Yang, PhD
|
1:00 PM – 1:30 PM |
First Principles of Global Leadership David Flores, MBA, Founder and CEO, BioCentury |
|
1:30 PM – 2:00 PM |
Genome Reading and Writing in CNGB Xun Xu, MBA, President, BGI Research Institute |
|
2:00 PM – 2:30 PM |
Meeting the market needs and creating sustaining value – the LakePharma story Hua Tu, PhD, Founder & CEO, LakePharma |
|
2:30 PM – 2:45 PM |
Sponsor Session: Gold sponsors
|
Afternoon Panel Session I: Developing Innovative Therapeutics in China
Session Chair: Xiaoli Qin, PhD, and Wei Huang
|
2:45 PM –3:30PM |
Moderator, Cheng Liu, PhD, CEO, Eureka Therapeutics Panelist, Michael An, PhD, Founder and CEO, Curegenix Panelist, Wei Zheng, PhD, Founder and CEO, Suzhou Connect Panelist, Baofu (Paul ) Ni, PhD, P, Kelun Bo-Tai Biopharmacetical Co. Ltd., and Senior Director of Kelun Pharmaceutical Research Institute Panelist, Tao Huang, PhD, JD, Venture Partner, Cenova Capital |
|
3:30 PM – 3:55 PM |
COFFEE BREAK |
Afternoon Session II: Investment and Entrepreneurship
Session Chair: Dannis Chang, PharmD, and Shenshen Hu, PhD
|
3:55 PM – 4:05 PM |
Sponsor Session: Gold sponsors
|
|
4:05 PM – 4:35PM |
Genomic Medicine and Modern Biotechnology Revolution: My Three Stories Dongliang Ge, PhD, CEO, Apostle, Inc. |
|
4:35 PM – 5:30 PM |
Afternoon Panel Discussion II: E-Club: Entrepreneurship and Investment Trends in Life Sciences Moderator, Alexis Ji, PhD, MBA, Illumina Ventures Panelist, Vincent Xiang, PhD, MBA, Partner, 6 Dimensions Capital Panelist, Karen Liu, PhD, Managing Partner, 3E Bioventures Panelist, Julie Papanek, MBA, Partner, Canaan Partners Panelist, Asish Xavier, PhD, MBA, VP, Venture Investments, JNJ Panelist, Lu Zhang, MBA, Associate Director of Business Development, Ascentage Pharma Panelist, Johnny Sun, MS, Co-founder and CIO, AccuraGen |
|
5:30 PM |
CONFERENCE ADJOURNED |
|
5:30 PM – 8:30 PM |
Post-Conference Reception Sponsored by Chengdu Medical City (Engage Room and Courtyard) Session Chair: Sihong Zhou and Ken Zhang, PhD |
CABS would like to express our sincere gratitude to all our sponsors for supporting us:

Limited Sponsorship Opportunities Still Available !
Email: fundraising@cabsweb.org
Questions? Email: info@cabsweb.org
San Mateo Marriott, 1770 South Amphlett Blvd, San Mateo, CA 94402
Announcing 2017 CABS K. Fong Award in Life Sciences
Chinese American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that Dr. Yinxiang Wang and Dr. Edgar Engleman are the winners of its CABS K. Fong Award in Life Sciences for their extraordinary achievements in research, entrepreneurship, and innovation. We will honor Dr. Wang and Dr. Engleman in the award ceremony at the 2017 BioPacific Conference (19th Annual Meeting of CABS) on June 24th, 2017 at the San Mateo Marriott San Francisco Airport, 1770 South Amplett Blvd., San Mateo, CA 94402.
Dr. Yinxiang Wang is the CEO and Chief Scientist of Zhejiang Beta Pharma, Inc. He is a member of the Chinese Program of Global Experts (also known as the “1000 Plan”). In his current position, Dr. Wang, is instrumental in the research and development of a great number of new drugs for oncology and diabetes.
As the Chief Scientist and one of the main innovators at Zhejiang Beta Pharma, Dr. Wang headed the research team which developed and commercialized Icotinib Hydrochloride (Conmana®), a National Category 1 Novel Drug that is the first small molecule oncology drug specifically targeting cancer cells that is completely developed in China. Zhejiang Beta Pharma successfully obtained approval on Icotinib Hydrochloride from Center for Drug Evaluation at SFDA at the end of 2010 and started marketing Conmana® in 2011.
Dr. Wang is also one of the founders of Zhejiang Beta Pharma New Drug Research Center in Beijing, established in 2003. Currently, he is leading the scientists at the center in researching and developing pipeline drugs that include eight Category 1 drugs and several Category 3 drugs, out of which five generic products have already been approved and commercialized.
Dr. Edgar Engleman received his bachelor’s degree from Harvard University and his M.D. from Columbia University. After completing training in internal medicine (UCSF), biochemistry (NIH), immunology, rheumatology and transfusion medicine (Stanford), he joined the faculty of Stanford Medical School where he is Professor of Pathology and Medicine. At Stanford, he supervises a large research laboratory in immunology, teaches the main tumor immunology course, directs the Stanford Blood Center which he founded in 1980, and co-directs the Tumor Immunology Program of the Stanford Cancer Institute. He is a managing partner of Vivo Capital, a firm that he co-founded in 1996 that invests in biomedical companies worldwide and has offices in Palo Alto, Beijing, Shanghai and Taipei.
At Stanford, Dr. Engleman spends much of his time teaching students about the immune system and overseeing the immunology research of his students. He has supervised more than 100 research trainees, authored more than 300 scientific articles and has been an editor of multiple scientific journals. The goal of his research is to discover new ways to direct the immune system against cancer and other life-threatening diseases. Twenty-five years ago he began testing the concept at Stanford that patients with cancer could be immunized against their own tumors through the use of powerful immune-stimulating cells known as dendritic cells. In 1992 he co-founded Dendreon where his technology provided the basis for the Sipuleucel-T (Provenge) prostate cancer vaccine, the first active immunotherapy for cancer to be approved by the FDA. This vaccine opened the way to a new era in which immunotherapies are increasingly becoming a standard component of cancer treatment. Recently, Dr. Engleman’s lab discovered a promising new immunotherapy that activates dendritic cells in tumors without requiring their prior removal and activation in vitro. This treatment strategy was shown to eradicate a wide range of tumors in mouse models (Nature 521:99-104, 2015) and the findings led to the formation of Bolt Biotherapeutics in 2016. His group has also developed a powerful new method that uses mass cytometry to analyze immune cells in multiple tissues throughout the body (Science 349:1259425, 2015), and by applying this new method, his group successfully identified critical parameters required for effective cancer immunotherapy (Cell 168:487-502, 2017).
About CABS K. Fong Award in Life Sciences
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria will be based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
· Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or the research reagent/equipment markets.
· Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
· Significant contribution to the CABS community and to promoting international collaboration in life sciences.
About Dr. Kenneth Fong
Dr. Kenneth Fong is a successful entrepreneur, an investor and mentor to many biotech founders, a community leader supporting higher education and a philanthropist. Currently Dr. Fong is the Chairman of Kenson Ventures, LLC, a venture capital firm that specializes in venture financing and strategic consulting to biotech companies. He is the founder and former CEO of Clontech, which developed and marketed Molecular Biology products to academic and pharmaceutical laboratories in the US and thirty other countries. He sits on the Boards of numerous biotech companies and serves on the advisory board of biotech-related entities in the US, China, Taiwan and Hong Kong.
Dr. Fong is active with both philanthropic and community activities. For example, he and his wife were major contributors in establishing the Fong Optometry and Medical Library at UC Berkeley, the Fong Scholarship Programs / Annual Scientific Symposia at San Francisco State University, the Annual Fong-Clontech Seminar series at Indiana University. His Community services included Chairman of the San Jose Chinese Performing Arts Center (2000-02), lead supporter to the Chinese Historical Society of America (SF, 2000) and the pioneering Biotech Program at the San Jose Tech Museum (1999). Recently, he has become more supportive of the educational programs for children in under-served and impoverished regions of China. He is a member of Committee 100 and a former member of the Board of 80-20 Initiative.
Currently Ken spends a good amount of time cultivating and advising a number of entrepreneurs (40-55 years old group) to grow their biotech companies.
Past recipients of CABS K. Fong Awards
2016: Dr. Gerald Chen, co-founder of Morningside, for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies.
2015: Dr. Irving Weissman, of Stanford University, for his pioneering research in stem cell research.
2014: Dr. Ge Li, Founder and CEO of Wuxi Apptec, for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Abbott for his leading role in the discovery of life- saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth, Plexxikon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.
online
2017 Hangzhou overseas entrepreneurial contest is calling for entrepreneurial projects for the competition! You are cordially invited to submit your project for this contest! The official language is Chinese. The submission deadline is June 30. For detail, please visit: http://tinyurl.com/2017hzc-CABS .
There are many entrepreneurial competitions in US, why taking the one from Hangzhou? To know the answer, please visit: https://sites.google.com/site/hzds2017
The contest will set up a total of 500 million yuan fund to support funding the winners of this contest.
Through previous two contests, more than 200 contestants have expressed their intentions to start a company at Hangzhou, while more than 50 companies from the contestants have officially registered and started running there , with the total VC funding of more than 300 million yuan.
Hangzhou, a place letting you achieve the dream of entrepreneurship!
杭州,创业者的梦想天堂
盛情邀请海外各路科技精英、创业英豪,前来报名参加2017杭州海外创新创业大赛!详情请浏览:
https://sites.google.com/site/hzds2017
【2017创业大赛报名方式】
大赛参赛项目报名采取网上登记和邮件补充提交其它材料的方式,请有意回国创业的海外精英尽早提交项目报名。报名截止期6月30日。报名时请注明是通过哪个协会或其它渠道知道这个大赛信息的,但请不要通过不同的渠道重复报名,谢谢配合。
报名网址: http://tinyurl.com/2017hzc-CABS
【投资界和企业界大佬们纷纷加盟2017杭州创业大赛】
丹华资本创始人-斯丹福大学张首晟教授将担任本届大赛北美赛区的海外总导师,出席并担任硅谷决赛和杭州总决赛的评委。他创立的丹华资本已发展成为硅谷最大的华人风险投资基金。详情请浏览: https://goo.gl/g8wnnF
自2015年首届大赛成功举办以来,参赛项目中将近40%属于“大健康”范畴,涉及医疗制药、基因工程、医疗器械、医疗信息化平台、健康服务等多个专业领域。为此,今年的大赛,竭力邀请到中国生物医药行业领军型企业——贝达药业股份有限公司作为合作单位。详情请浏览: https://goo.gl/zwSeZN
汽车行业领军企业——吉利集团成为大赛合作单位! 浙江吉利控股集团始建于1986年,1997年进入汽车行业,多年来专注实业,专注技术创新和人才培养,取得了快速发展。现资产总值超过千亿元,连续五年进入世界500强,连续十四年进入中国企业500强,是中国汽车行业十强,是国家“创新型企业”和“国家汽车整车出口基地企业”。浙江吉利控股集团总部设在杭州,旗下拥有吉利汽车、沃尔沃汽车、伦敦出租车等品牌。现有帝豪EC7(A级轿车)、博瑞(B级轿车)、博越(SUV)、帝豪GS(跨界SUV)、远景系、金刚系等10多款整车产品及1.0L-3.5L全系列动力总成产品。详情请浏览: https://goo.gl/TvZIMn
【杭州,一个可以让你施展才能,实现创业梦想的地方】
到杭州创业的十大理由请浏览: https://goo.gl/9insyM
前两届大赛得奖项目请浏览: https://goo.gl/A2hcl1
【这么多创业大赛, 为什么要参加杭州的大赛?】
实际落地率高, 政府补助和扶持力度大。大赛评比公平, 评委由海外和国内风投专家组成,现场实时打分和公布结果。2015年和2016年前两届大赛已有200多个参赛项目达成落地意向,50多个项目正式落地,注册资金达3亿多元。2015年首届一等奖获得者王孟秋博士的“计算机视觉与智能控制融合技术”项目,获市政府500万元资金资助和风投基金—“浙民投”1500万美金投资,仅用1个月时间就正式落户浙江未来科技城(海创园),注册资本1个亿。中央电视台新闻联播和焦点访谈都对该项目进行了连续报道。第二届一等奖获得者江涛博士“高性能骨再生医学材料”项目也已于年前落户高新区(滨江)。大部份得奖项目已在杭州落地, 并得到政府500-1500万RMB的无偿补助及办公空间补助。而且有不少得奖项目是已经在国内其它地区落地有公司的。
参加杭州海外创业大赛也可以大大提高风投和潜在大客户对项目的关注度; 很有可能在参加大赛过程中,让潜在客户更深入地了解了项目的产品优势而产生合作机会。
和往届相比,本届大赛将更加着力于整合各类资源,着力打造由政府主导、海外社团组织举荐、企业及风投机构对接,集合政策、资金、行业、市场、人才等各类创新创业资源在内的高端综合性创新创业平台。通过海外导师制度、大赛项目特别对接渠道、大赛项目对接综合平台的对接扶持,结合加强对项目产品市场适应性和竞争力的关注的具体评判方式,加上风险投资基金和政府扶持政策的先后投入,意图使更多掌握核心创新技术、具备产业化前景、具有较强市场竞争力的创新创业项目能够通过大赛平台获得发展所需的各类资源,在杭成立企业迅速成长。
【大赛奖励及扶持措施】
大赛将成立总额 5 亿元人民币的“创客天下”大赛风投基金,用于参赛项目融资对接。
进入大赛决赛的项目可获得1.5万元人民币的优胜奖金。
北美赛区和欧洲赛区共产生12个总决赛项目。大赛总决赛(杭州)设一等奖1个,二等奖4个,三等奖7个,获奖项目可分别获得20万元、10万元和5万元人民币的奖金。
获得大赛一、二、三等奖、分赛区决赛7-11名、分赛区决赛12-18名及进入复赛阶段的优秀项目,于大赛结束一年内来杭落地实施转化的,可不经评审分别获得300-500万、200万、100万、50万、20万元人民币的市级高层次留学回国人员在杭创业创新项目资助资金。
【大赛时间】
大赛从2017年4月启动,7月海选,8月复赛和9月决赛,到11月“2017浙江∙杭州国际人才交流与项目合作大会”期间到杭大赛总决赛结束。项目收集后,北美和欧洲赛区通过海选评各选出30-50个左右创业项目入围复赛。然后通过视频答辩的方式,各评选出18-20个创业项目入围决赛。北美赛区决赛将于9月底在美国硅谷举办,决出6个项目进入总决赛的参赛。邀请进入总决赛的参赛项目持有人或团队代表将参加在“2017浙江·杭州国际人才交流与项目合作大会”会议期间举办大赛总决赛。
【参赛对象及要求】
参赛对象应熟悉中文,原则上要求在海外取得硕士及以上学位,具备海外创新创业工作经验,拥有市场前景广阔的创新性技术或成果,目前在北美地区且有意向来杭创新创业的海外高层次人才。已在杭州落地创新创业的人员及其团队成员不再作为参赛对象。
【参赛要求】
参赛者可以以个人或团队形式参加。团队要求成员间的专业结构合理,具有关联性和互补性,可稳定合作3年以上。
每个参赛个人或团队只能申报一个参赛项目。
参赛项目中所提出的产品和服务,可以是参赛者参与或经授权的发明创造或专利技术,也可以是一项可能研发实现的概念产品或服务。项目产业属于高新技术产业和战略性新兴产业,产品、技术有较好的先进性。参赛团队核心成员必须对其参赛项目拥有合法的知识产权或使用权(授权)。
参赛个人或团队应在进行广泛市场调研和分析的基础上,制作将产品或服务推向市场的完整、具体、有实施可能的商业计划书,其中应包括项目的技术、市场前景、经营策略、资金需求等方面,并展示团队的成员和未来规划,商业模式清晰。
【参赛目的】
大赛以发展杭州市信息经济、推动智慧应用为重点,通过整合创业资源,提升项目质量,强化行业对接,吸引更多的海外人才和项目来杭创新创业,助推杭州市经济转型升级。
盛情邀请海外各路科技精英、创业英豪,前来报名参加2017杭州海外创新创业大赛!
如愿意成为大赛宣传的合作单位,帮助征集或推荐项目报名的科技协会社团和高校校友会, 请联系
北加州浙江大学校友会
Presentation via Video Conference (视频答辩)

Root for the CABS Dragons (team photo) as they paddle (video) with timing, technique, teamwork, and toughness at the Annual Foster City Novice Dragon Boat Race! You'll get a taste of the following:
- Learn from a coach, who is a veteran paddler and medaled at the Club Crew World Championships
- Discuss the history of how this cultural Chinese folk ritual evolved into a world-class sport
- Practice the art and science of team-building while having fun with CABS
Register by Saturday, June 10th:
- Spectator registration ($0) to receive updates (e.g., tent location, race schedule, parking map) via WeChat using this QR code to join the group
- Dri-fit jersey / souvenir ($16) to show your team spirit and wear it with pride (will be sold at the CABS tent on race day)

For questions and/or volunteer opportunities, please email Jenny Liu (Jenny[dot]Liu[at]cabsweb.org).
--------------------------------------------------------------
If any company is interested in sponsoring this initiative for marketing opportunities (e.g., corporate-branded jerseys, food promotions, team registration fee support), please email fundraising@cabsweb.org.
Leo Ryan Park Lagoon in Foster City, CA
ChinaBio® Partnering Forum 2017
May 31 – June 1, 2017
Zhuhai, China
https://ebdgroup.knect365.com/chinabio-partnering/
.png)
ChinaBio® Partnering Forum has grown to be the premier life science partnering conference in China. Now in our 9th year, 2016 attracted over 900 decision makers from around the world, including nearly 300 from the US and Europe and 600 from China. For the first time, on May 31 - June 1, 2017, the event will be held in Zhuhai, an emerging hub of life science in Southern China. Zhuhai is committing significant funding and resources to the life science industry, including building the state-of-the-art Jinwan Biopharmaceutical Park, with 250,000 m2 of incubator, R&D and manufacturing facilities. The ChinaBio conference features EBD Group’s partneringONE® system, enabling delegates to efficiently arrange 1-1 meetings with companies from across the life science value chain. Join us at ChinaBio® Partnering Forum 2017 May 31 - June 1, to meet 100s of biotech and pharma leaders from around the world along with hundreds of China-based developers of novel technologies for two days of productive partnering in Zhuhai.
We offer all CABS members 20% discount on regular registration fee. Please use CABS20 as the promo code.
If there is any question, please email at fundraising@cabsweb.org
Zhuhai, China
Dear CABS members,
Thank you for all those that made the last commercial workshop such a great success. The session sparked a very lively and productive conversation which I hope was helpful to all those in attendance (I certainly learned quite a bit!)
In reviewing feedback following the first session, we noticed a common interest in understanding the levers and frameworks for valuation, especially as it pertains to commercial attractiveness to potential investors. Therefore, we are going to tailor the second workshop to foster a discussion around patient-based forecasting. The drivers and inputs into constructing a patient-based forecast will be a great starting point in thinking about the commercial viability of medical products.
Hope to see you all on Sunday, and thanks again for your support and participation.
Sincerely yours,
Anthony Hsiao
CABS Business and Career Development Committee
Registration: free for CABS members; $20 for non-member or join CABS today and get this event for free!
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010

Enrollment Reopens for 2016-2017 CABS Entrepreneur Club (E-Club)
By popular demand, we are reopening the enrollment for the 2016-2017 CABS Entrepreneur Club (E-Club) to enable more CABS members to join the exciting monthly events at our E-Club !
About CABS E-Club:
Aligning with our mission of catalyzing the professional interactions of our members in the life sciences industry, CABS is very pleased to launch our CABS Entrepreneur Club (CABS E-Club 2016-2017) to foster the innovative entrepreneurial spirit.
We are very honored that Dr. Alexis Ji, Partner at Illumina Ventures is our Chair of CABS E-Club (2016-2017). The CABS E-Club is jointly hosted by the International Collaboration Committee (ICC) and Business & Career Development (BCD) Executive Councils.
2016-2017 Past CABS E-Club Events:
- TURNING INNOVATION INTO SUCCESSFUL BUSINESS: STORY OF ADVANCED CELL DIAGNOSTICS AND KENSON VENTURES.
Speakers: Yuling Luo, Ph.D., Founder, President, and Chief Executive Officer, Advanced Cell Diagnostics & Kenneth Fong, Ph.D., Founder and Chairman of Kenson Ventures, LLC
Sponsor: Morrison Foerster
- IMPORTANT FACTORS FOR SUCCESS CAREER AND ENTREPRENEURSHIP IN BIOTECH.
Speaker: Dr. Jing-Shan “Jennifer” Hu, Ph.D., Partner Qiming U.S. Healthcare Ventures
Sponsor: Hanqi Investment
- 2017 E-CLUB KICKOFF
Organizer: Alexis Ji, Ph.D., MBA, Partner, Illumina Ventures
Sponsor: Hanqi Investment
- ENTREPRENEURSHIP - FROM IDEA TO PRACTICE.
Speaker: Guo-Liang Yu Ph.D., Executive Chairman, Crown Biosciences; Venture Partner, OrbiMed Asia
Sponsor: Jones Day
- DRAGONS & UNICORNS: CAPTURING STARTUP OPPORTUNITIES AND AVOIDING HIDDEN PITFALLS
Speakers: Karen K. Wong, Ph.D., J.D., Partner & David Yi Wang, Associate
Sponsor: Wilson Sonsini Goodrich & Rosati

It is our pleasure to inform you that Annual Biopharma Development and Production China Week, which consist of 3 co-located events namely, 7th Annual Biomanufacturing Conference, 6th Annual Cell Line Development and Engineering Asiaas well as 8th Annual Biosimilars Asia, is taking place this May 16 – 17 at Hilton Shanghai Hongqiao Hotel.
As the Biopharma industry grows in China and the Asian region, we invite you to be part of this tremendous growth and development opportunities!
IBC’s Biopharma Development & Production Week in China is THE meeting place for biopharma industry professionals and scientists to get the highest quality and practical information that will enable them to develop competitive advantages and advance their capabilities in developing and manufacturing cell lines, biosimilars, biobetters, antibodies, vaccines or novel biologics.
Click here for detailsWith the VIP code P46326CABS, CABS members can enjoy 20% off normal rates for the events under BDP China
Hilton Shanghai Hongqiao Hotel

Career Advisory Network (CAN) is a mentoring program, designed to help advance career goals of working professionals, postdocs, graduate students in biotech/pharma industry. It offers a six-month program with 1:1 mentoring meeting, brunch gathering, and group network sessions. Launched in 2010, CAN has been successfully held for past six years and facilitating an interactive and productive relationship where mentees and mentees feel valued and valuable. For the details of the program, please check CAN website: http://www.cabsweb.org/CAN
The deadline for mentee application will be May 25th, 11:59 pm.
2017 Mentor list: 2017-CAN-Program-Mentor-Bio.pdf
Mentee Application link: here
For sponsorship, volunteer opportunity, mentor application, or any other questions/comments, please contact bcd@cabsweb.org
Online

To foster team-building (videos - 1 and 2) and express your interest in joining the team (pending availability), please complete this poll HERE.
Paddlers were selected based on the following:
- Attendance on race day and three practices (5/21, 6/4 and 6/10 from 3 - 4:30PM) pending the team's availability HERE
- Gender due to the minimum requirement of 8 women
- Capacity of a boat holds 20 paddlers with 2 alternates on the team roster
If selected, please pay online for the following to reserve your seat by Saturday, April 29th:
- Registration fee ($20) will be sponsored by CABS for current and new members, who join HERE
- Dri-fit jersey / souvenir ($16) to show your team spirit and wear it with pride without chafing
For questions and/or suggestions, please email Jenny Liu (jenny[dot]liu[at]cabsweb.org).
------------------------------------------------------------
If any company is interested in sponsoring this initiative for marketing opportunities (e.g., corporate-branded jerseys, food promotions), please email fundraising@cabsweb.org.
Leo Ryan Park Lagoon in Foster City, CA

Chinese-American Bio-Pharmaceutical Society (CABS) and Department of Postdoctoral Associate of Chinese Students and Scholars at Stanford (ACSSS) sincerely invite you to join the 2017 ACSSS|CABS Career EXPO.
Please register at the eventbrite link to meet employers on May 7th:
https://2017careerexpo.eventbrite.com/
When: May 7th (Sunday), 12:30 pm - 6:00 pm
Where: LKSC LK101/102 (Career Fair), LK120 (Registration and Coffee Talk), 291 Campus Drive, Stanford, California 94305
Free Parking: Roth way garage (Campus drive @ Roth way)
Schedule
12:30 -1:00 pm: Registration and Vendor setup
1:00 - 2:45 pm: Career Fair (LK101/102) and Q&A Booths (LK120)
2:55 - 4:15 pm: Coffee Talk (LK120)
- · 2:55 – 3:00 pm: Greeting from CABS and ACSSS
- · 3:00 – 3:15 pm: Keynote: Ziang Hu, Ph.D. Huawei Fellow, Vice President of Software Lab | Huawei R&D USA
- · 3:15 – 3:20 pm: Industrial presentation: BitTiger
- · 3:20 – 3:35 pm: Keynote: Frank Zhong, Ph.D | VP of Advanced Engineering R&D | BGI
- · 3:35 – 3:40 pm: Industrial presentation: Shanghai Henlius Biotech, Inc.
- · 3:45 – 3:50 pm: Industrial presentation: Predicine Inc.
- · 3:50 – 3:55 pm: Industrial presentation: Qlife Lab
- · 3:55 – 4:10 pm: Keynote: Rhea Daugherty, M.Ed, Ph.D | Associate Director | Stanford Medicine Career Center
- · 4:10 – 4:15 pm: Industrial presentation: Genentech
4:20 - 6:00 pm: Career Fair (LK101/102) and Q&A Booths (LK120).
The event will feature over 30 companies from biotech, pharma, IT, and energy industry. Below is a list of confirmed employers
Gilead Science Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. Gilead's portfolio of products and pipeline of investigational drugs includes treatments for HIV/AIDS, liver diseases, cancer, inflammatory and respiratory diseases, and cardiovascular conditions.
Morrison & Foerster is a firm of exceptional credentials. Our name is synonymous with a commitment to client service that informs everything that we do. We are recognized throughout the world as a leader in providing cutting-edge legal advice on matters that are redefining practices and industries.
Kelun Pharmaceutical is the market leader of injectable and infusion technologies. We are committed to R&D for small-molecule NCEs and biological NBEs, biosimilars, generic drugs, and new drug delivery systems (NDDS) in oncology, diabetes, hepatitis, cardiovascular and other major disease areas.
Wuxi AppTec, established in December 2000, is a leading global pharmaceutical, biopharmaceutical, and medical device open-access capability and technology platform with global operations. Our services are designed to help customers shorten the discovery and development time, lower the cost of drug and medical device R&D through cost-effective and efficient solutions.
Predicine, founded in Silicon Valley, CA in 2015, is focusing on the next generation liquid biopsy diagnostic technique. We have established an integrated "one-stop-shop" biomarker service platform, and we are conducting big data analysis to enable precision medicine in personalized cancer care and global drug development.
LakePharma is a leading contract research organization (CRO) specializing in antibody and protein engineering, cell line development, and protein production. Services can be fully integrated, creating a "soup to nuts" solution for all of a customer's molecular biology, cell line, and protein needs.
Shanghai Henlius Biotech, Inc. specializes in the discovery and development of innovative monoclonal antibody therapeutics, bi-specific antibodies and antibody drug conjugates (ADCs) by using the state-of-the-art technologies. Our core mission is to make quality antibody-based therapeutics available and affordable to all patients in need worldwide.
Chia Tai Tianqing Pharmaceutical Group with the largest R&D and manufacturing base for liver disease drugs in China, is an innovative pharmaceutical group that engages in drug R&D, manufacturing and marketing. We are honored as one of the National Key High and New Tech Enterprises, and ranked 25th in National Top 100 Medical and Pharmaceutical Powers in 2013.
Nvigen Inc. develops multifunctional and biodegradable nanoparticles. Our proprietary nanoparticle-imaging-delivery (NID™) technology and our expertise in nanochemistry comprise the engineering platform for our research and development.
BGI was established in 1999 with participation in the original Human Genome Project. Now as one of the world’s largest genomics organisations, BGI is experienced in genomic research, commercial NGS services and sequencer manufacturing. We are committed to providing solutions to address the research, pharmaceutical, and clinical markets.
Genentech is a leading biotechnology company that discovers, develops, manufactures and commercializes medicines to treat patients with serious or life-threatening medical conditions. We are among the world's leading biotech companies, with multiple products on the market and a promising development pipeline.
Boehringer Ingham is the largest privately held pharmaceutical corporation in the world and ranks among the world's 20 leading pharmaceutical corporations. Headquartered in Ingelheim, Germany, we focus on researching, developing, manufacturing and marketing new medications of high therapeutic value for human and veterinary medicine.
Axbio Inc
Apostle is a biotechnology start-up headquartered in Sunnyvale, CA. We are in the business of the research, development, licensing, and sales of novel AI (Artificial Intelligence)-Enabled Nanotechnologies and the related intellectual properties, products, and services for diagnosis and treatment of human diseases.
Huawei is a leading global information and communications technology (ICT) solutions provider. We have established end-to-end advantages in telecom networks, devices and cloud computing. We are committed to creating maximum value for telecom operators, enterprises and consumers by providing competitive solutions and services.
RoboteX, founded by alumni of PayPal and YouTube, RoboteX is a Silicon Valley-based technology company that is bringing a unique approach to the robotics market. RoboteX designs robots for safety and ease-of-use from the ground up, and we work directly with end users to include capabilities that are important to them.
BICI USA Co., LTD is established in Silicon Valley (San Francisco, CA) with the goal of providing maximum resources to innovators and startup businesses interested in China.
Grandall law firm, was initially formed upon the amalgamation of Beijing Zhang Yongtao Law Firm, Shanghai Wanguo Law Firm and Shenzhen Tangren Law Firm, and it was then registered with the Ministry of Justice of the People’s Republic of China. Grandall is one of the largest trans-regional partnership law firm in China.
Snackoo, is a company that endeavors to connect people with tasty snacks from all around the world. Snackoo intends to build a multi-channel and multi-category brand in the snack industry. We seek to fulfill every hungry moment with satisfaction.
GCP, is a global talent service firm. We provide services including executive search, consulting, and event planning.
BitTiger, is a life-long learning platform from Silicon Valley. BitTiger provides quick, effective, and affordable training in practical skills that prepare students to be as productive as possible in their current and future jobs.
Leetcode, is one of the best code learning platform helping students and engineers to land on their dream jobs.
JD.com American Technologies Corporation: JD.com is the largest e-commerce company by revenue in China, with recent plans to strengthen our leadership position as a technology-driven company with focus on big data, AI technology and smart logistics. Our JD Silicon Valley R&D Center focuses on developing and enhancing new and existing technologies to improve user experience and expand the company’s offerings.
Sohu.com, is China's premier online brand and indispensable to the daily life of millions of Chinese, providing a network of Web properties and community based/Web 2.0 products which offer the vast Sohu user community a broad array of choices regarding information, entertainment and communication.
Suning: Suning International USA was established in 2016 to integrate all Suning branches and resources in the United States which includes Suning R&D Center, Global Procurement Center and Investment Center.
SVC Angel, is a platform for cross-border investment, co-investment, collaboration, co-marketing. We are managed by Silicon Valley veterans with deep root in industry and investments. We invest in intelligent system including artificial intelligence, computer vision, NLP, big data, smart manufacturing, sensing technology, control technology, low power devices, etc.
UCAHP, is a Silicon Valley-based platform for high-level professionals, and an engine for innovation and entrepreneurship. UCAHP was founded by successful entrepreneurs with exceptional experiences in growing startups to IPOs, bridging US and China markets, investing smart money as professional VCs with entrepreneur background.
WE Career, Located in Silicon Valley, WE Career provides Online-to-Offline professional human resources services, targeting Chinese-English hybrid talent and China-Affiliated Corporations. Our APP is one of the largest career platforms in the US, with access to more than 300,000 Chinese working professionals and students. We have successfully held more than 25 large-scale career fairs.
BeyondSoft, Founded in 1995 and headquartered in Beijing, Beyondsoft is a global IT Consulting, Solutions and Services provider. We provides powerful solutions and products for clients in high-tech, eCommerce, finance, automobile, retail, logistics, energy, manufacturing, healthcare, telecommunications, media & entertainment, and travel.
Qlife Lab, founded in 2013, is a global biotechnology company developing HT-mass spectrometry based omics technologies and solutions to drive clinical diagnosis. With headquarter in Shenzhen China, 7 divisions across China, and R&D centers in California and Missouri, we offer novel metabolomics-based laboratory diagnostic tests that may improve early detection of diseases.
We welcome employers and sponsors to join us. Please contact fundraising@cabsweb.org
As a valued sponsor of the event, you will be able to:
- Promote your company to > 3000 CABS members who are all part of the life sciences industry and >3000 scholars/students from Stanford University
- Promote your company through the alliance network of CABS and ACSSS
- Reach out to hundreds of potential employees from entry level to senior level.
- Find top-tier talents for your open positions across different healthcare practices
Golden, silver sponsorship categories are available, enabling your organization to benefit in different levels of exposure as part of event advertising and promotional activities.
|
BENEFIT |
GOLD |
SILVER |
|
5 minutes featured talk |
* |
|
|
Vendor Stand |
* |
* |
|
Logo on promotion materials |
* |
* |
|
1 month job posting on CABS website |
* |
* |
Sponsorship: fundraising@cabsweb.org
Event details: bcd@cabsweb.org
Li Ka Shing Learning and Knowledge Center, LK101/102/120
Immuno-oncology beyond checkpoint inhibitors
If any company is interested in sponsoring, please contact fundraising@cabsweb.org.
Please register online. Online registration is free for CABS members and $10 for non-members. You can become a CABS member and register online free. Onsite registration is $10.
When register, please enter your affiliation and name on your photo ID which is required at onsite check-in.
1:00-1:30PM check-in, onsite registration and networking
1:30PM-4:30PM
Presentations:
1. After CD19: CAR-T for solid tumor

Cheng Liu, Founder and CEO, Eureka Therapeutics
Dr. Cheng Liu is the founder and CEO of Eureka Therapeutics, a California biotech company dedicated to antibody research and discovery for cancer immunotherapy. Prior to founding Eureka, Dr. Liu was a Principal Scientist in antibody drug discovery at Chiron (now Novartis), where he championed anti-CSF1 antibody program for treatment of bone metastasis to human clinical trials. He is the inventor of multiple issued US patents in drug discovery. In 2007, he was awarded Special US Congressional Recognition for his contributions to improving human health. Dr. Liu received his B.S. in Cell Biology and Genetics from Beijing University and a Ph.D. in Molecular Cell Biology from the University of California, Berkeley.
2. Targeting patient-specific neoantigens using a personalized, Live-Attenuated Double Deleted Listeria monocytogenes (pLADD) Immunotherapy

Chan C. Whiting, Director of Immune Monitoring and Biomarker Development, Aduro Biotech
Dr. Chan Whiting is currently the Director of Immune Monitoring and Biomarker Development at Aduro Biotech. She received her Ph.D. at UCLA in the Department of Microbiology and Immunology where her thesis focused on identifying and characterizing novel signal transducers involved in cytokine and T cell receptor (TCR) signaling pathways. She did post-doctoral training in chemokine biology at Amgen followed by research into E3 ligases in the development of autoimmune diseases at Stanford University. Prior to joining Aduro Biotech, Dr. Whiting has worked for systems biology companies including Entelos, Inc. and Ingenuity Systems (currently Qiagen) in developing mathematical mechanistic models and other in silico computational tools for target discovery and validation for autoimmunity, infectious diseases and oncology. She applies systems-based technologies along with traditional immune assays towards biomarker development efforts in immune oncology at Aduro.
3. Oncolytic viral immunotherapy for the treatment of advanced cancers

Caroline J Breitbach, Vice President, Translational Development, Turnstone Biologics
Caroline J Breitbach is Vice President, Translational Development at Turnstone Biologics, focusing on the development of an oncolytic viral immunotherapy for the treatment of patients with advanced cancers. Caroline was previously Vice President, Clinical & Translational Research at SillaJen Biotherapeutics (formerly Jennerex Biotherapeutics), where she oversaw Phase 1 and 2 clinical studies an oncolytic vaccinia virus. Caroline received her undergraduate degree at McGill University in Montreal, Canada. She subsequently joined Dr John Bell’s group at the University of Ottawa in Ottawa, Canada where she evaluated acute effects of oncolytic viruses on the tumor immune microenvironment.
Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080
Guangzhou University Faculty Recruitment Program
International "1,000 Young Talents Plan" Forum
May 20 - 22, 2017
Guangzhou, China
Forum Purpose
This forum will gather outstanding scholars from China and abroad to participate in an academic symposium and exchange at Guangzhou University to locate outstanding faculty who are eligible to be hired for the “1,000 Young Talents Plan”.
Forum Travel and Accommodations
Guangzhou University will provide invited scholars with financial aid to include travel and accommodation expenses.
Registration Deadline
The registration deadline is May 5, 2017.
Qualifications, Application and Further Information
Please see the attachments for details.
Guangzhou, China
CAN Program Kick Off Event For CABS Members
Saturday April 29, 2017 @ 1:00 pm – 3:30 pm
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Registration on EventBrite:https://can_2017.eventbrite.com
Dear CABS members,
It is my great pleasure to invite you to the 2017 CABS Career Advisory Network (CAN) program Kickoff event. CAN is a six-month program with 1:1 mentoring meetings and group network sessions. We have lined up an exceptional group of mentors for 2017 with diverse expertise and experiences encompassing pharmaceutical R&D, biotechnology development, project management, business development, patent law, management consulting, venture capital and entrepreneurs. Once matched, mentees will meet with their mentor monthly to discuss specific career-building topics. As part of the process, participants will create a personal strategic plan for identifying ways to achieve career goals. This year, we will be collaborating with Chinese Biopharmaceutical Association (CBA) to expand the CAN program, in which we will increase the size of our mentorship pool from both the mentor and mentee perspective. This will broaden the wealth of mentorship expertise and foster a culture of collaboration and partnership with local associations in the Bay Area, in addition to the commitment we have for our members from both organizations.
CAN program has been successfully held for the past seven years and received many positive feedbacks. It is an excellent opportunity to interact and network with a diverse group of people in academia and industry in a supportive and informal setting from both CBA and CABS mentors. 2017 CAN program will continue to provide our members an interactive relationship with mentors discussing personalized career issues such as work life balance, transition alternative career path, entrepreneurship in US and China, workplace interpersonal skills and managerial skills.
Join our kickoff event to learn more about the program details and network with mentors and other mentees. You will have the opportunity of identifying a mutually agreed-upon mentor or mentee at the meeting. Seats are limited. The online application link and mentor list will be published CABS CAN Program website after the 4/29 kickoff event.
Best,
Dannis Chang and Shenshen Hu
Business and Career Development Committee
Registration Requirement: Free to members and non-members. Must be a member of either CABS or CBA to participate as a mentee.
Please register here: https://can_2017.eventbrite.com
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010

April. 12, 2017
CABS Introduces Global Life Sciences Programs to Serve Singapore
San Francisco, CA, USA - (April 12, 2017) – CABS is very pleased to announce that we will introduce our global programs to serve the Singaporean life sciences community.
Headquartered in the San Francisco Bay Area, CABS is one of the most influential non-profit life sciences organizations in the United States and China.
Our initial focus in Singapore is to provide the Singaporean life sciences community access to our global network and introduce flagship programs such as our Career Advisory Network (CAN), a mentorship program launched eight years ago in the United States, Entrepreneur Club (E-Club), an exclusive networking group to connect entrepreneurs and investors, Science Workshops, and BioPacific Conference held by CABS annually in the San Francisco Bay Area.
“Our vision is to serve as a gateway between the life sciences communities in Pacific Rim countries,” commented Dr. Cheni Kwok, President of CABS. “By working closely with our advisors from local life sciences companies, global firms supporting life sciences, universities and research institutes at the Agency for Science, Technology and Research (A*STAR), we strive to foster new collaboration opportunities and exchanges between the life sciences community in Singapore and our current members in San Francisco Bay Area and China.”
“Associations such as CABS provide avenues for continuous learning, opportunities to build a stronger professional community amongst employees of biomedical companies, and help entrepreneurs gain access to global networks and membership. Today, Singapore is home to more than 50 biomedical sciences manufacturing plants, producing a wide range of products ranging from drugs to medical devices and equipment, with a combined manufacturing output of approximately $26 billion and employment of more than 19000 in 2015. In tandem, we have a growing number of successful local enterprises in the biomedical sciences industry across medical devices, biopharmaceuticals and digital health. We welcome CABS’ engagements in Singapore and are committed to developing the industry in the years to come,” said Ms. Weng Si Ho, Director, Singapore Economic Development Board.
“We strive for building a strong membership base in Singapore, connecting local members to their peers in US and China, and promoting cross-border innovation and entrepreneurship. We hope to collaborate with local organizations and host more local events to better serve our members in Singapore in the near future” said Simon Cen, Chair of CABS Singapore.
Our introduction event will be held at 7pm-9pm on April 24, 2017. The event is free and open to all life sciences professionals, entrepreneurs and investors in Singapore. Members of the interested public and media are also welcome to attend.
About CABS:
The Chinese American Biopharmaceutical Society (CABS) is a non-profit organization for professionals in the life sciences industry, headquartered in San Francisco, California. We are currently serving over 3000 members and subscribers in US, China and Pacific Rim countries. We organize year-round programs including scientific workshops, business forums, mentorship program, social and networking events. CABS is the proud organizer of the BioPacific Conference, a highly anticipated annual life sciences event in the San Francisco Bay Area.
|
CABS Introduction Event in Singapore Date: 24 April 2017, Monday Time: 7:00 PM – 9:00 PM Venue: Level 2, 60 Biopolis Street #02-01 Genome, Singapore 138672 Agenda |
||
|
7:00 PM |
Introduction of CABS |
Simon CEN Chapter Chair, CABS Singapore |
|
7:20 PM |
Keynote Address Life Sciences Innovation in Singapore |
Prof. NG Huck Hui Executive Director, Genome Institute of Singapore, A*STAR |
|
7:40 PM |
Keynote Address Data Science for Biomedicine and Healthcare |
Dr. Lawrence WEE Chief Data Scientist, Zuellig Pharma |
|
8:00 PM |
Plenary Session Ways to help Singapore Life Sciences Professionals to Thrive |
CABS Advisors and Mentors |
|
9:00 PM |
END |
Online registration is required to attend the event. Please register below.
Contacts:
Simon Cen
Chapter Chair, CABS Singapore
Ee Fann Goh
Chapter Secretary, CABS Singapore
Level 2, 60 Biopolis Street #02-01 Genome, Singapore 138672
CRISPR TECHNOLOGY AND APPLICATIONS
If any company is interested in sponsoring, please contact sponsorship@cabsweb.org.
Please register online. Online registration is free for CABS members and $10 for non-members. You can become a CABS member and register online free. Onsite registration is $10.
1:30-2:00PM check-in, onsite registration and networking
2:00PM-4:00PM
Presentations:
1. Benjamin Haley, PhD, Scientist, Genentech, Inc.
During his graduate studies, Ben unraveled several biochemical mechanisms that characterize the RNAi pathway. After receiving his PhD, he went to Mike Levine’s lab at UC Berkeley as an American Cancer Society Postdoctoral Fellow. There, he developed an enhanced Drosophila RNAi system for in vivo applications, further refined the in vivo specificity of RNAi, and identified novel aspects of small RNA biogenesis and mRNA structure/expression using a host of model organisms. Following his postdoc, Ben joined Genentech, Inc. where he currently serves as a point person for applied functional genetics. His lab is focused on developing technologies to better model human disease and therapeutic intervention using cell-based experiments.
Talk title: CRISPR platform overview and functional genomic screening for drug target discovery and validation
2. Dr. Ruby Chen-Tsai 陈雁如, PhD, Co-founder and Chief Scientific Officer, Applied StemCell, Inc.
Dr. Chen-Tsai received her PhD from Cornell University and did her post-doc research at Stanford University. She has been working with stem cells and genetic cell and animal models for 25 years. Prior to joining Applied StemCell, Dr. Chen-Tsai worked at Stanford University for 16 years as the Director of Transgenic Research Center and Associate Director of the Stanford Cancer Institute, overseeing nine research technology labs. Dr. Chen-Tsai is a co-inventor of the TARGATT™ integrase technology and a related “DICE” technology for site-specific gene insertion. Her current research focuses on stem cell based gene therapy using most advanced genome editing technologies including CRISPR/Cas9 and Applied StemCell, Inc.’s proprietary TARGATTTM technologies. Dr. Chen-Tsai is an author of many scientific papers published in Science, PNAS, Genetics, Cell Stem Cell, and etc. and holder of several patents. She is an awardee of several NIH grants, California Institute of Regenerative Medicine grant, and Invest Northern Ireland government grant.
Title: New CRISPR technologies and complex genome engineering for discovery and gene therapy.
Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080
Dear CABS members,
It is my great pleasure to invite you to the first Commercial Strategy Workshop. The mission of these series of workshops is to provide an overview of the business decision making process in the commercializing of new therapeutics and medical devices. These workshops will be valuable to anyone interested in learning more about the business side of life sciences and potential career paths.
The first workshop will cover the tools and methodology of opportunity assessments and determining market fit for a new therapeutic or medical device. Specifically, we will be covering:
- Life science commercial strategy framework
- Primary market research methodology
- Secondary data analytics methodology
- Market maps
- Patient journeys and buying process
Topics for future workshops will include marketing tactics, forecasting, performance management, and other topics of interest to the CABS membership.
Sincerely yours,
Anthony Hsiao, MBA
CABS Business and Career Development Committee
Registration: free for CABS members; $20 for non-member or join CABS today and get this event for free!
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010

Are you interested in elevating your career development by taking it to the next level? If so, here are some examples of how Toastmasters International can help improve your leadership and communication skills:
- Scientists can enrich presentations with the right amount of context, detail, and visual aids for various stakeholders
- Entrepreneurs can practice pitching with storytelling techniques that illustrate how their technology transforms industry
- Business professionals can enhance the impact of their talks by engaging audiences with body language
To learn more, please complete the following:
- View the Toastmasters club experience with this video HERE
- Share your input for a CABS Toastmasters club via this poll HERE
- Review the roles HERE to become a meeting participant and/or Club Officer
- Register to attend the mini-meeting (including a speech on how to inspire your audience) demonstrated by the Toastmasters staff
Let's overcome America's #1 fear of public speaking together among fellow CABS members while talking about various topics, networking, and having fun!
For questions and/or suggestions, please email Jenny Liu (jenny[dot]liu[at]cabsweb.org).
Hanqi Investments (1633 Bayshore Highway, Suite 280, Burlingame, CA 94010)
THE DEFINITIVE PROTEIN & ANTIBODY ENGINEERING SUMMIT IN CHINA
As China rises to join the ranks of global biopharmaceutical powerhouse, the true test lies not only in their regulatory guidance and pathways, but also in their successful research and development of novel biotherapeutics. Industry leaders and visionaries will convene at the Fourth Annual PEGS Summit in Shanghai, China on March 28-30, 2017 to share innovative discovery programs, advancements in cutting-edge technology, best practices and experiences in problem solving.
NEW for 2017 With the growing importance and significance of immunotherapy as a potential game-changer in the fight against cancer, we have added a new track on “Immuno-Oncology” which will showcase the pipelines and progress of immunotherapy programs of Chinese and international companies.
http://www.pegsummitchina.com/
Shanghai, China
Using the Immune System to Treat Cancer

Monday, March 27, 2017
6:00pm - 9:00pm
AbbVie
1500 Seaport Blvd, Redwood City, CA 94063
CABS member get $10 off using code "327ISTC"
Register here
Immuno-oncology is the hottest area of cancer research and drug development, with recent successes having spawned a modern-day gold rush of activity. Join the MITCNC Life Science Forum to learn about this important area of medical research.
The concept of destroying cancerous tumors by harnessing the immune system dates back to 1891, when Dr. William Coley observed some benefit by injecting patients with toxic bacteria. However, it has only been recently that therapies targeting the immune system have proven clinically successful.
Lawrence Fong, MD, is the Efim Guzik Distinguished Professor in Cancer Biology at the UCSF Helen Diller Family Comprehensive Cancer Center where he leads the Cancer Immunotherapy Program. He is also Co-Director of the Parker Institute of Cancer Immunotherapy @ UCSF.
Dr. Fong obtained his BA from Columbia and MD from Stanford. He completed internal medicine training at the University of Washington, and oncology fellowship and post-doctoral training at Stanford. He then moved to UCSF as an assistant professor in 2002.
He is an elected member of Alpha Omega Alpha and the American Society for Clinical Investigation. He has served on multiple NIH study sections and committees including the NCI Investigational Drug Steering Committee (IDSC), and NCI Genitourinary Cancers Steering Committee (GUSC). He has also served on multiple editorial boards including the Journal of Clinical Oncology, Journal of Immunotherapy of Cancer, and Cancer Immunology Research.
AbbVie, 1500 Seaport Blvd, Redwood City, CA 94063
Change of venue with more capacity, and open for new registration
This event is very popular. If any company is interested in sponsoring, please contact sponsorship@cabsweb.org.
Please register online. Online registration is free and will be closed when the limit is reached. After that you will be put on a waiting list. Onsite registration is $10.
1:30-2:00PM check-in, onsite registration and networking
2:00PM-4:00PM
Presentations:
1. Liquid biopsies in precision drug development by Shidong Jia, Founder and CEO of Predicine Holdings Ltd.
Cancer immunotherapy and targeted therapy offer great promise in precision oncology, where predictive biomarkers have been extensively investigated in various types of cancer patients. The talk will describe development and clinical application of an innovative, next generation sequencing based liquid biopsy test to support cancer immunotherapy clinical trials.
Speaker bio:
Dr. Shidong Jia is the Founder and CEO of Predicine Holdings Ltd, an international precision medicine organization that is committed to developing innovative diagnostics, therapeutics and big data in cancer and other serious diseases. The company operates in California, USA and Shanghai, China. Driven by a passion to improve personalized medicine, Dr. Jia has dedicated the past 21 years to basic, translational, and clinical cancer research. His previous work identified p110beta as novel drug target for PTEN-deficient prostate tumors (Jia S, et al. Nature, 2008), a finding that laid the foundation for the clinical development and testing of p110beta inhibitors in cancers. Most recently, his team at Predicine developed world’s first ctRNA and ctDNA combined liquid biopsy test for precision medicine in cancer. A former Scientist, principal investigator and prostate cancer disease area lead at Genentech, Dr. Jia spearheaded the biomarker strategies and drug & diagnostics co-developments efforts in support of clinical oncology pipeline at various stages (i.e., early stage research, late stage research, early clinical development, Phase I and Phase II clinical trials), culminated by the Lifecycle Investment Point (LIP) achievement of driving an investigative drug from global Phase II into Phase III pivotal clinical trial. Prior to joining Genentech, Dr. Jia was Laboratory Head for Oncology Drug Discovery at the Novartis Institutes for BioMedical Research in Cambridge, MA. Dr. Jia serves as the Editor-in-Chief of "Journal of Circulating Biomarkers”, Guest Associate Editor of "Frontiers in Oncology", and Members of National Cancer Institute Review Committees and the Italian Ministry of Health - Scientific Research and Health Innovation Review Committee. Dr. Jia is a BayHelix member and receipt of several awards, including the "Young Investigator Award" at the Fourth Asian Congress for Microcirculation in Indonesia and the "P.A.R.T. Investigatorship Award" at the Dana-Farber Cancer Institute, Harvard Medical School in Boston.
2. Towards an Assay of Global Immunocompetence by Holden Maecker, Director, Human Immune Monitoring Center, Stanford
A long-term goal of our laboratory is to define metrics of healthy immune function and the deviations that predict or define disease states. There are many facets of the immune system that can be measured, and few clinically accepted tests of immune function. However, we hypothesize that a comprehensive assessment of the proportions, phenotypes, and functions of specific immune cell subsets in blood should be helpful in defining these metrics. We have therefore defined for this purpose a mass cytometry assay using phorbol myristate acetate (PMA)+ionomycin stimulation of peripheral blood mononuclear cells (PBMC), with staining for approximately 40 cell-surface and intracellular proteins, including lineage markers, cytokines, and other functional markers. Mass cytometry, or CyTOF, is a version of flow cytometry that uses heavy metal ion labels in place of fluorochromes, with readout by time-of-flight mass spectrometry. The CyTOF platform allows for many more labeled antibodies to be used in combination, with much less spillover between detection channels, compared to conventional flow cytometry. In this talk, I will describe this methodology and how we are applying it currently to the monitoring of patients undergoing cancer immunotherapy. Our goal in these studies is to better tailor immunotherapies to specific patients, and to suggest approaches that might be useful to address those patients with suboptimal immune function.
Speaker Bio:
Dr. Holden T. Maecker received his PhD from Stanford University, is currently Assoc. Prof. at Microbiology & Immunology department and Director of Human Immune Monitoring Center at Stanford. His research is in the area of T cell response signatures and their association with protection from chronic pathogens and cancer. His lab has published a number of papers on this topic, both alone and in collaboration with other groups, in systems such as CMV, HIV, tuberculosis, and cancer. The Human Immune Monitoring Center (HIMC) is a Stanford core service laboratory, which performs blood processing and standardized assays using flow cytometry, genomics, and immunoassay platforms. For the past five years, his lab has gained extensive experience with CyTOF mass cytometry. In addition to being PI on multiple NIH, industry, and foundation grants, Dr. Maecker serves as a core leader for several cooperative center grants and other large collaborative projects, in which he oversees the use of HIMC assays, including data analysis and interpretation.
Thanks to our sponsors: Predicine and SeqMatic
Genentech Building 31, 310 DNA Way, South San Francisco CA, 94080

Click here to Register Now!
EPPICon 2017
11th Annual flagship event of EPPIC Global
Keynote Speakers
Sanjaya Kumar, MD, MPH (Synepta Group, Inc.)
Since 1998, EPPIC Global has been serving the San Francisco Bay Area life sciences community promoting entrepreneurship, networking, partnering, and mentoring. Join us to hear expert talks and panel discussions onImmunotherapy, Bioinformatics and Devices
- If targeted therapies and personalized medicine revolutionized cancer treatment in the last decade, willImmunotherapy be the next big hope during the coming decade?
- Have we finally got a handle on big data and where is Bioinformatics headed?
- Ditto with regard to Biomarkers and Devices?
Advances in cancer immunotherapy have generated enthusiasm across all fields of oncology. Complimentary/companion diagnostics along with predictive biomarkers are integral to improving patient outcome. In addition, emphasis on personalized medicine and targeted therapies, diagnostics and biomarkers are playing an increasingly important role in drug development. Early adoption of bioinformatics tools can help advance therapeutic candidates.
EPPICon 2017 continues the grand tradition of bringing you the latest advances and opportunities in science and technology.
- Get updated on the state of the art in science and business
- Form new alliances
- Explore investment opportunities
Where
Crowne Plaza Hotel San Francisco Airport
1177 Airport Blvd, Burlingame, CA 94010
Who Should Attend
Entrepreneurs, Innovators, Researchers, Investors and Professionals (including Service Providers) from Pharma, Biotech, Diagnostics, Devices and Digital Health
OPEN for Registration NOW!!!
Registration online: $100
On-site Registration: $150
Student/Post Doc Registration Online: $75* (Valid student ID to be presented at Conference)
EPPIC Charter Members** / Sponsors** / Event Speakers**
(Registration includes food and beverage - breakfast, lunch, coffee breaks and evening reception)
* Student/Post Doc promo code "STCON17"
** Renew or Register with Code provided to you: Conference is complimentary
Visit www.eppicon.org for more information
*************************
Program Outline
8:00 AM: Registration open - Breakfast and Networking
8:30 AM: Opening Remarks
9:00 AM: Opening / Plenary Keynote - Ira Mellman, PhD, Genentech
9:45 AM – 4:45 PM: Panels and Speakers
- Immuno-Oncology Panel: Chan Whiting, PhD, Aduro Biotech, Robert Sikorski, MD, PhD, Five Prime Therapeutics, Caroline Breitbach, PhD, Turnstone Biologics, Alex Franzusoff, PhD, PACT Pharma
- Bioinformatics Keynote Speaker: Nikesh Kotecha, PhD, Parker Institute for Cancer Immunotherapy
- Bioinformatics Panel: Arshad Ahmed, Philips, Richard Bourgan, PhD, Genentech, Bevan Emma Huang, PhD, Janssen R&D (J&J), Nikesh Kotecha, PhD, Parker Institute for Cancer Immunotherapy
- Devices: Nikhil Bhat, PRS Medical Technologies, Katie Anderson, Verb Surgical, Inc.
- Speed Pitch (Please see below for details)
4:45 PM: Closing Keynote – Sanjaya Kumar, MD, MPH, Managing Director, Synepta Group, Inc.
5:30 PM: Evening Reception and Networking
**************************
EPPICon 2017: Speed Pitch Session
Speed Pitch: Entrepreneurs in Life sciences pitch their ideas to seasoned investors - a unique opportunity for symbiosis of ideas and investmentsWhat is the Need for Speed Session
This is a fabulous opportunity for start-ups get on stage and make a five-minute pitch to a distinguished panel of top-notch VC’s and biotech executives. Multiple entrepreneurs of early stage companies in the life-sciences can talk about their idea, technology, market, etc., get quick feedback from the VC panel and get exposure to various potential collaborators / partners.Selected presentations will get highlighted on the EPPIC website and other promotional material.
Why present at the Need for Speed Session
This conference attracts more than 200 professionals in the life science industry. The conference attendees are experienced life-sciences professionals in the R&D, Service and management careers. Our members are distributed across therapeutics, informatics, devices, and diagnostics - the ideal audience for early stage companies.What’s in it for Entrepreneurs:
- Get VC's interested in your idea.
- Attract advisors, mentors
- Attract business, technical partners
- Get constructive feedback and potential reinforcement of technology.
What do you need to do in five minutes.
Prepare an elevator pitch. You have just five minutes so please focus on the most important aspects of your company or technology.- Mission statement
- Product opportunity and market potential
- State of the company, management team, development timelines and key milestones
- Key differentiating factors from existing products and how they meet market need
- What is the entrepreneur looking for – specifically clearly articulate funding goals, use of proceeds and potential milestones achieved with this funding
What, When, Where
Please email a presentation to ramani@shasta-bioventures.com by March 10, 2017, to be considered for selection. Shortlisted candidates will be notified by March 17, 2017.
EPPICon 2017 Organizing Committee
www.eppicon.org
EPPIC GLOBAL
www.eppicglobal.org
eppic@eppicglobal.org
(408) 357-0601
| Click to view this email in a browser If you no longer wish to receive these emails, please reply to this message with "Unsubscribe" in the subject line or simply click on the following link:Unsubscribe |
|
EPPIC GLOBAL PMB# 402, 4546 El Camino Real Suite B-10 Los Altos, California 94022-1069 US Read the VerticalResponse marketing policy. |
Crowne Plaza Hotel San Francisco Airport, 1177 Airport Blvd, Burlingame, CA 94010
This event is very popular. If any company is interested in sponsoring, please contact sponsorship@cabsweb.org.
Please register online. Online registration is free and will be closed when the limit is reached. After that you will be put on a waiting list. Onsite registration is $10.
1:30-2:00PM check-in, onsite registration and networking
2:00PM-4:00PM
Presentations:
1. Personalized health care for oncology by Craig Cummings, Associate Director, Oncology Biomarker Development, Genentech
2. NGS application in immunology studies by Naibo Yang, Director, Immunogenetics, Complete Genomics Inc.
The Role of Next-Generation Sequencing in Enabling Personalized Oncology Therapy
In the 15 years since the publication of the draft human genome, sequencing technologies have advanced rapidly and the cost to sequence a genome has dropped precipitously from several billion dollars down to several thousand dollars. NGS now enables analysis of a large number of DNA bases in a cost- and time-effective manner. In recent years, this promising technology has been transferred to clinical diagnostics in oncology with the aim to better define which therapy should be given in each patient. The aim of this tutorial is to review next-generation sequencing (NGS) techniques and the emerging role of this technology in the diagnosis and treatment of cancer. I will provide a basic overview of the different types of NGS and the strengths and weaknesses of each approach in the context of cancer as a heterogeneous disease driven by DNA-altering mutations. Finally, I will discuss how this information can inform personalized clinical development of targeted therapies.
[Review article is available online at http://onlinelibrary.wiley.com/doi/10.1111/cts.12429/full]

Craig Cummings, PhD
Associate Director, Oncology Biomarker Development
Genentech, a Member of the Roche Group
Dr. Craig Cummings received his PhD in Genetics and pursued a postdoctoral fellowship in Microbiology and Immunology at the Stanford University School of Medicine. He is currently Associate Director of Data Science in the Genentech Department of Oncology Biomarker Development, where his group is responsible for biomarker data management, analysis, and development—in collaboration with Foundation Medicine—of NGS-based methods for cancer immunotherapy diagnostics. Prior to joining Genentech, he contributed to development of next-generation sequencing platforms, tools, and diagnostic tests at Applied Biosystems/Life Technologies and Sequenta, Inc.
High Throughput Sequencing and Its Applications on Immunology
High Throughput Sequencing is rapidly penetrating all aspects of biological research, from genomics, genetics to oncology, immunology, aging, general health management etc. Complete Genomics Inc, as a BGI company, is focusing on high throughput sequencing technologies and its applications. Within this large scope, my team studies the immune system with NGS technology. The total collection of immunoglobulin molecules (BCRs or antibodies) and TCRs are collectively called immune repertoires. They are ideal subjects for NGS studies. Through a single sequencing study, millions of unique antibody and TCR sequences could be obtained. Their status is important to one’s health. And their responses are key components of body’s fighting against diseases, regardless dealing with invading pathogens or cancer.
This talk will mainly focus on NGS and its applications in immunology field. It will cover topics of immune system and health, immune repertoire studies through NGS , antibody discovery research and cancer immune therapy etc.

Naibo Yang, PhD
Director of Immunogenetics
Complete Genomics Inc ( A BGI company)
BIO: Dr. Naibo Yang received his PhD in Physiology from Thomas Jefferson University and got his postdoc training at Stanford University. He is currently carrying on his research at both Complete Genomics (Mountain View) and BGI headquarter (Shenzhen), as the Director of Immunogenetics. His research is focusing on NGS applications in immunology and novel antibody discovery technologies. Prior to Complete Genomics, Dr. Yang had extensive experience in drug discovery and diagnostics. He was a major contributor to discovery research at Exelixis Inc and Affymax Inc, in the fields of oncology and blood disease related therapeutics. Besides his scientific research activities, Dr. Yang is also a longtime contributor and supporter of CABS.
Genentech Building 31, 1531 Grandview Drive South San Francisco CA, 94080
Welcome to join the family of CABS E-Club! It's so exciting to see so many friends share the passion about entrepreneurship, and it's my honor to serve you as the chair of E-club in 2017. I am excited to let you know that we are going to have the E-club KICK-OFF meeting this coming Saturday March 11 between 3-5pm PST.
E-Club Kick-off Meeting:
Date: March 11, Saturday, 3:00-5:00pm PST
Location: Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Please try your best to come to this first E-club meeting, where you will have a chance to:
- Introduce yourself to your fellow E-club members and start the networking to find new friends, partners and collaborators
- Learn about the planned activities of E-club and CABS; More importantly, brainstorm with the whole group to share your thoughts on the activities you would like the E-club to organize in 2017
- Volunteer to lead events and activities at E-club in 2017
Please sign up on this google spreadsheet if you plan to come by Friday Mar 10 so that we can prepare enough refreshments.
E-Club Wechat Group:
I've started a WeChat group for "CABS E-Club" where we will use for future events announcement and group communication. Please scan the QR code at the bottom of this message to join the wechat group.
Other CABS activities:
There are a few other CABS events and activities being organized that I want to draw to your attention:
- CABS Cross-Border Business Roadshow (CBBR), April 16th, 2017, Sunday 6-9pm PST
-
- A series of business pitch sessions to local and China investors via teleconference. CBBR provides a platform for CABS members to pitch their businesses to potential investors and collaborators, including Pharmaceutical Executives, Venture Capitals, Private Investors, Incubators and Industrial Parks.
- CBBR is an invitation-only event, ~50 attendees in all locations, i.e. San Jose (onsite), Beijing (video Call) and Shanghai (video call). We would like to invite you to present at CBBR. The first session will be held at Hanhai Investment Inc (San Jose office) on April 16th, 2017, Sunday 6-9pm PST (April 17th, 2017 Monday 9am-12pm Beijing time).
- To register for this event and for more information, please contact Xiaoli Qin (xlchin@gmail.com) before March 31th with the title of your talk and a brief introduction of your project (non-confidential information only).
- CABS Toastmasters Club Poll survey
-
- CABS is planning a Toastmasters Club. CABS will cover the new member fee for the first 20 charter members as an investment that helps you better plan and execute your exciting list of activities for the new year.
- The CABS Exec Council needs your quick input on Toastmasters in order to proceed. Please take a quick poll of five quick questions (< 5 min.) survey
- More information about the ToastMaster Club:
-
- Description of all roles (including officers): http://bit.ly/2iQFH7l
- Kickoff date: March 2017 (TBD)
- Venue: Hanqi Investments (1633 Bayshore Highway, Burlingame, CA 94010), 2nd Floor
- Response deadline for poll: Sunday, March 19th
- Please contact Jenny Liu (jenny.liu@cabsweb.org) for more info.
Look forward to seeing all of you on Saturday 3/11 at the E-club Kick-off meeting!
Best regards,
Alexis Ji,
Chair, CABS E-Club 2017
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
TOPICS:
1. "Tax Preparation" by Dr. Wen Fang Liu & Ms Michelle Chao, Deloitte Tax LLP
2. "Financial and Tax Planning for a Peaceful Retirement" by Dr. Connie Chen, TransAmerica Financial Group
Ticket Info:
https://www.eventbrite.com/e/caspa-financial-seminar-i-tax-preparation-tickets-32037454890
If there are any questions, please call (408) 428-9988.
ITRI North American Office 2870 Zanker Road #109 San Jose, CA 95134
Why is the Tri-Conference the Premier Event on Molecular Medicine & Diagnostics?
If you are working in diagnostics and drug discovery, this is
the must attend event of the year.
Attracting over 3,500 drug discovery and development
professionals from over 40 countries in 2016, the Tri-
Conference has grown into a diverse event, focusing on
Molecular Medicine, specifically on Discovery, Genomics,
Diagnostics and Information Technology.
Why attend the Tri-Conference?
- HEAR over 500 speakers from across all industries, all research fields, and from all over the world
- CHOOSE from over 400 presentations and panel discussions
- NETWORK with 3,500 drug discovery and development professionals from over 40 countries
- SHOWCASE your Research by Presenting a Scientific Poster (and save $50!)
- PARTICIPATE in one of 30 Roundtable Discussions
- VIEW over 170 Scientific Posters
- VISIT with over 200 companies in the exhibit hall
Moscone North Convention Center, San Francisco, CA

Due to party room conflict, the Chinese New Year party will be changed to the following new location:
Burlingame Parks & Recreation, 850 Burlingame Avenue, Burlingame, CA 94010
Sorry about this change. If you already registered but cannot attend due to location change, we will refund your registration fee. Email publication@cabsweb.org for refund.
The old year leaves amidst the falling snow 瑞雪纷飞辞旧岁 The new spring comes with the shining glow 旭日东升迎新春
Happy Chinese New Year!!! Please join us on Sunday, January 29, 2017, to kick off the Year of the Monkey with exciting CABS traditional New Year Festivities!
11:30 AM - 1:00 PM: Chinese New Year Lunch
1:00 PM - 4:00 PM: CABS Chinese New Year Gala
Program to include: Singing and dancing; Beijing opera; Live musical entertainments; Spring festival couplets and conundrum, and much more!
There will be games for children and lucky draws! Many prizes are waiting for you! Come to meet old friends, make new friends and have fun! Let’s get together to celebrate year of the rooster!
We sincerely appreciate our sponsors for their generous support
Registration Requirement:
On-Line:
Member: $10; Non-Member: $15; Kids, 12 and under, seniors: $2
On-Site:
Member: $15; Non-Member: $20
Kids, 12 and under: $2; Seniors: $8
When you register on line, please specify the numbers of Children and Seniors whom you will bring in as guest registration and pay a flat fee of $2 per person. This will help us prepare the right amount of food. Boxed lunch may not available if not registered on line!!!
Thanks to the sponsors of this event:

Gold Sponsor:
Andy Gan, Ph.D., Realtor
Berkshire Hathaway CA Realty
650-922-3168
Andy@AndyGan.com
www.PhdRealtorBlog.com
Ranked top 1% nationally, Andy provides value real estate service to home owners and buyers in the Peninsula. Andy has been the No. 1 agent at Berkshire Hathaway CA Realty for 4 consecutive years from 2013 to 2016. In 2016, Andy sold over 40M in Foster City and had a total sales volume of 60M in the Peninsula. Andy specializes in residential and investment purchase and is a certified relocation specialist. Andy also services non-resident individual buyers and corporate clients from China including arranging mortgage and business loans. Andy and his real estate group also service listings in South Bay, East Bay, and San Francisco.
Gold Sponsor:
Jessica Yau
Better Homes and Gardens Real Estate, JFF
1116 South El Camino Real San Mateo, CA 94402
Jessica Yau has been one of the top agents in Better Homes and Gardens Real Estate consistently since 2011 and award recipient in the prior years with Prudential California Realty since 2004.
Her determination and drive, combined with her straight-forward communication style and masterful knowledge of the local market, all make Jessica the premier choice for your real estate needs.
Silver Sponsor:
李瑞中医针灸院 (LI'S TCM HEALTH CENTER)
联系: Tel or Text to(650)815-1203
E-mail AcupunctureLi@gmail.com

Sponsor:
FCCAA

Burlingame Parks & Recreation, 850 Burlingame Avenue, Burlingame, CA 94010
BGI 2017 International Talent Acquisition Program – Silicon Valley Info Session
(and business development opportunity)
华大基因2017国际人才招聘计划 – 硅谷宣讲会
(及商业拓展机会)
Please register at your earliest convenience. Registration is free.
Agenda
6:00 pm - 6:20 pm Networking
6:20 pm - 6:30 pm Introduction and welcome by CABS
6:30 pm - 7:00 pm Business Landscape and Career Development at BGI, by Ye Yin, Ph.D., CEO of BGI Genomics
7:00 pm - 7:30 pm R&D and Global Talent Demand at BGI, by Xun Xu, Ph.D., Director, BGI Research, Executive Director, China National GeneBank
7:30 pm - 7:45 pm BGI's Journey of Globalization, by Ning Li, Ph.D., Chief Development Officer, BGI Genomics
7:45 pm - 8:15 pm Panel Discussion
8:15 pm - 8:30 pm Networking
Event Description
The executive team of BGI (formerly known as Beijing Genomics Institute) will be joining CABS and ACSSS for recruitment and information session at Stanford LK130 on Jan 13, 2017. The delegation consists of Dr. Ye Yin, the CEO of BGI Genomics, Dr. Xun Xu, Executive Director of BGI Research and the Chinese National GeneBank, Dr. Ning Li, Chief Development Officer of BGI Genomics. They will give a high profile overview of BGI’s history and vision in global health, the current R&D and business scope both in China and the international space as well as their personal career path with the growth of BGI. This event also serves as a kick-off of BGI’s 2017 global talent recruitment program in the US. We welcome any interested parties and individuals to join the event and to discuss career/business opportunities brought from BGI.
BGI 2017 International Talent Acquisition Program
Job location: US: Mountain View, Boston, Philadelphia; China: Shenzhen, Hong Kong, Tianjin, Wuhan
Job Level: Senior Individual Contributor, Director level
Job Function: R&D, Product Development, Business Development
The detailed job descriptions will be send out before the info session. Stayed tuned!
Speaker Bio

Ye Yin. Ph.D.
CEO, BGI Genomics Co. Ltd.
Dr. Ye Yin, serves as Chief Executive Officer of BGI Genomics Co., Ltd. He leads his team providing total genomics solution to top medical centers, global pharmaceutical companies and institutions. Ye has rich experience in global business management, international collaboration and lab production management. He was the crucial contributor to SARS diagnosis in 2003 and also a key member of “1000 Plant and Animal Reference Genomes”. He has published over 50 scientific papers on most prestigious academic journals like Nature, Science and PNAS, etc. Ye actively involves in lots of non profit organizations to help fund HLA and other rare disorder diseases.

Xun Xu, Ph.D.
Director, BGI Research, Executive Director, China National GeneBank.
Dr. Xun Xu serves as Director of BGI-Research and Executive Director of China National GeneBank (CNGB). He leads the technology development in the area of molecular biology, information technology, assembly and evolution of plants and animals, plant and animal genetic polymorphism, animal and plant molecular breeding, single cell sequencing platform, microchip-based DNA synthesis technology and synthetic genomics. He led a team of BGI Research to successfully develop the sequencer, BGISEQ-500, which is now the only commercial sequencing platform designed and made in China. In addition to sequencing technologies, he also led the cutting-edge experimental and bioinformatics developing at BGI. He has published 139 papers in high impact international journals and holds over 50 patents. He was awarded the youngest Senior Researcher in Natural Science and Technology of Hubei Province in 2013, the “National Leading Talent” in Shenzhen City in 2014 and the "Grand Challenge Young Scientists" by the Chinese Ministry of Science and Technology and Bill & Melinda Gates Foundation in 2015.

Ning Li, Ph.D.
Chief Development Officer, BGI Genomics Co. Ltd.
Dr. Ning Li currently serves as the Chief Development Officer at BGI Genomics Co. Ltd., responsible for BGI’s international business as well as its global development on BGI community of precision medicine. He was the CEO of BGI Europe from 2011-2015 and has led this BGI subsidiary from 5 employees to over 100 today by successfully forming key business partnerships and scientific collaborations with private and public institutions across Europe. Prior to that, Dr. Li led a research group within BGI Shenzhen, which successfully developed NGS-based epigenomics platforms and bioinformatics analysis tools specifically for epigenome data analysis. He received his Ph.D. in Bioinformatics at Beijing Institute of Genomics of the Chinese Academy of Science in 2010, and his Bachelor of Physics from Nankai University in 2004.
About BGI
BGI was founded in 1999 with the vision of using genomics to benefit mankind and has since become the largest genomic organization in the world. With a focus on research and applications in the healthcare, agriculture, conservation, and environmental fields, BGI’s distinguished achievements have made a significant contribution to the development of genomics throughout the world. BGI also offers a wide portfolio of transformative genetic testing products across major diseases, enabling medical providers and patients worldwide to realize the promise of genomics-based diagnostics and personalized healthcare. BGI’s services and solutions are available in more than 62 countries around the world.
Headquartered in Shenzhen, BGI started international operations in 2009 and now has a global presence throughout the Americas, Europe, and Asia-Pacific, and includes both non-profit and for-profit parts. BGI has established 47 labs (including joint labs) globally. While its largest international laboratory is in Hong Kong, BGI also has major facilities in Copenhagen, Denmark; Mountain View, California, and Philadelphia, Pennsylvania. BGI has over 5000 employees worldwide.
Organizer
- BGI (华大基因)
- CABS (Chinese American Biopharmaceutical Society)
- ACSSS (Association of Chinese Students and Scholars at Stanford)
Co-organizers and medium support:
- CBA (Chinese Biopharmaceutical Association)
- ACSS at UCSF (Association of Chinese Students and Scholars at UCSF)
- CASPA (Chinese American Semiconductor Professional Association)
- CGPSA (Chinese Graduate and Postdoctoral Scholars Association at UC Berkeley)
Recommended Parking Garage: Pasteur Visitor Garage A (200 Pasteur Dr)
Contact Information
Frank Hu, Ph.D.
Business Development Manager – Bay Area
BGI Americas Corp.
Yan Wang, Ph.D.
Committee Chair – CABS Allance Management
fundraising@cabsweb.org
Room 130 Li Kai Shing Center, Stanford University (291 Campus Drive, Palo Alto, CA 94305)
2017 CABS Investor Forum
About the Event:
Back by popular demand, CABS is very pleased to announce that the 2017 CABS Investor Forum will be held on January 11, 2017 (Wednesday), in conjunction with JP Morgan Healthcare Conference 2017 in San Francisco.
Chinese investments in US businesses hit a record of $15B last year, with California remaining a top destination. Chinese direct investment in the US biotech and health sector has seen a dramatic increase recently. This event will celebrate the establishment of new multi-national investment funds for life sciences and successful fundraising by our entrepreneurs.
This forum, running from 8:00am-12:00pm, will feature two panel discussions joined by US/Asia healthcare investors and entrepreneurs, focused on discussing the US-Asia cross border healthcare investment trends, transactions, opportunities and challenges.Agenda:
8:00-8:55 Registration, Breakfast & Networking
8:55-9:00 Welcome Remarks by Cheni Kwok, Ph.D., CLP, President, CABS
9:00-9:15 The State of China Biotech: An Investment Perspective by Greg Scott, Founder, ChinaBio Group
9:15-10:15 Panel 1: China Healthcare Enterprises Going Global - Strategic Moves of Large Players
Moderator: Janet Xiao, Ph.D., J.D., Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Confirmed speakers:
Minqi Li, Investment Director, GF Securities-Bay City Capital
Scott Liu, Ph.D., CEO & Co-Founder, Shanghai Henlius Biotech, Inc.
Leidi Zhang, Managing Director, China Life Private Equity Investment Limited
Lianshan Zhang, Ph.D., Senior Vice President and Global R&D President, Jiangsu Hengrui Medicine
Howard Liang, Ph.D., CFO and Chief Strategy Officer, BeiGene
10:15-10:30 Networking Break
10:30-11:30 Panel 2: Bridging the Gap of Healthcare Innovation between US and China -- Opportunities and Challenges for Investors and Emerging Companies
Moderator: Alexis Ji, Ph.D., M.B.A., Partner, Illumina Ventures
Confirmed speakers:
James Huang, Managing Partner, Kleiner Perkins Caufield & Byers China
Judith Li, M.B.A., Partner, Lilly Asia Venture
Andrew Thompson, President & CEO, Proteus Digital Health
Michael Chang, M.B.A., Managing Director, Vivo Capital
Wei Zhou, Ph.D., J.D., President & CEO, Centrillion Bio
11:30-12:00 Networking
Speaker Biographies (alphabetical order)

James Huang, Managing Partner, Kleiner Perkins Caufield & Byers China
James Huang, M.B.A., joined Kleiner Perkins Caufield & Byers China as a managing partner in 2011 and focuses on the firm’s life sciences practice. His main investment interests are innovation around China’s growing healthcare markets and helping entrepreneurs build companies. James has made more than 15 investments in China since 2007.
Before coming to KPCB China, James was a managing partner at Vivo Ventures, a venture capital firm specializing in life sciences investments. While at Vivo, James led numerous investments in China. Before joining Vivo in 2007, James was president of Anesiva, a biopharmaceutical company focused on pain-management treatments. During his 20-year career in the pharmaceutical and biotech industry, he also held senior roles in business development, sales, marketing and R&D with Tularik Inc. (acquired by Amgen), GlaxoSmithKline LLC, Bristol-Meyers Squibb and ALZA Corp. (acquired by Johnson & Johnson). James is Chairman of Board at Kindstar Global, JHL Biotech and Director at GenScript, ChiralQuest, Zenesis, CVie Therapeutics, EntreMed, XW Laboratory and Omni Pharmaceuticals.
James received an M.B.A. from the Stanford Graduate School of Business and a B.S. degree in chemical engineering from the University of California, Berkeley. He was born in Taipei, Taiwan.

Alexis Ji, Partner, Illumina Ventures
Alexis Ji, Ph.D., M.B.A., is a partner at Illumina Ventures. She brings 12 years of experience in research and venture investment in the genomics and pharmaceutical industries. At Illumina Ventures, Alexis focuses on investing in early-stage genomics technology platforms and applications in life science tools, diagnostics, therapeutics, and digital health.
Prior to Illumina Ventures, Alexis was a principal at WuXi Apptec Corporate Venture Fund and then WuXi Healthcare Ventures, a U.S.-China cross-border life science venture capital firm, where she focused on investments in therapeutics and medtech companies in the U.S. Alexis was instrumental in investments in Twist Bioscience, 23andme, Juno Therapeutics, Unity Biotechnology, Vivace Therapeutics, Medeor Therapeutics, Ideaya Therapeutics, and Lumo Bodytech. Before joining WuXi, Alexis was a venture investment consultant at ARCH Venture Partners, where she evaluated early-stage life science and physical science opportunities and was involved in the founding of Twist Bioscience and the investment in Nextcode Health, and provided operating assistance to Boreal Genomics and PixelEXX Systems. Earlier in her career, Alexis worked as a senior scientist at Merck in early drug discovery, at Roche in virology translational research and clinical trial development, and at Life Technologies in molecular diagnostics and next-generation sequencing.
Alexis earned her Ph.D. in Molecular Genetics from Washington University in St. Louis, where her thesis was on non-coding RNA discovery. Alexis holds an MBA from University of Chicago Booth School of Business, and a Bachelor in Biochemistry from Shandong University.

Judith Li, Partner, Lilly Asia Venture
Judith Li, M.B.A., is a Partner at Lilly Asia Ventures (LAV), based in Hong Kong / Shanghai and focused on early and growth stage investments across biopharmaceuticals, medical devices, and diagnostics both domestically and cross-border.
Judith holds board appointments at a variety of LAV’s portfolio companies including Nextcure, Crown Biosciences, Just Biotherapeutics, and Veritas Genetics. Her prior experience includes McKinsey’s New York office, hospital administration at Partners Healthcare, and co-founding an interventional nephrology medical device venture.
Judith holds a B.A. in Neurobiology from Harvard and M.B.A. from Harvard Business School, and currently resides in Hong Kong with her husband Ben, splitting her time between Hong Kong, Shanghai, and the Bay Area.

Minqi Li, Investment Director, GF Securities-Bay City Capital
Minqi Li, CFA, is Investment Director of Bay City Capital GF XINDE Life Sciences USD Fund, a newly established life sciences VC fund jointly managed by GF XINDE and Bay City Capital. The fund invests in the broad life sciences sector, geographically in the US, Europe and China.
Minqi holds Master’s degrees in molecular medicine from Sun Yat-Sen University and genetics and genomics from Stanford University. She also holds a Master’s degree in management from Paris-Sorbonne University, France. Minqi obtained her bachelor degree in finance from Sun Yat-Sen University and got clinical medicine training in the Medical School of Sun Yat-Sen University.

Scott Liu, CEO & Co-Founder of Henlius Biotech Inc
Dr. Scott Liu, CEO & Co-Founder of Henlius Biotech Inc., graduated from Purdue University’s Department of Biology, was a postdoctoral fellow at Stanford University, and later pursued iMBA courses at Syracuse University. He has many years of experience in management and leadership. He has previously served as vice president of UBI and Bristol-Myers Squibb, and was also the QC director of Amgen. Because of his outstanding achievement, Dr. Liu has won the Bristol-Myers Squibb Company's Technical Operation Award as well as the Focus of Attention Award. In addition, he was also awarded the National Science Council of Taiwan Distinguished Investigator in 1993. Dr. Liu Scott has 20 years of experience in biopharmaceutical development, manufacturing, drug administration and quality management , and he won the title of " 1000 Planning Expert of Shanghai " in 2013.

Greg B. Scott, Founder and Chairman, ChinaBio® Group
Greg founded ChinaBio® Group in 2007 to help western life science companies achieve success in China. ChinaBio works with U.S., European and APAC companies seeking partnerships, acquisitions, novel technologies and funding in China. ChinaBio has helped identify over 1000 partnering, licensing and acquisition candidates, and raise over $500M in gov't and VC funding in China. ChinaBio also organizes the highly successful ChinaBio Partnering Forum which draws over 850 attendees from around the world each spring. Greg is also co-founder of two private investment groups that have funded over 50 biotechnology and medical device companies in the U.S. and China, and Executive Editor of ChinaBio® Today, the most widely read source for China life science news. Headquartered in Shanghai, ChinaBio® has staff in San Diego, Palo Alto, Cleveland, Toronto and Basel, Switzerland. Greg lives and works in Shanghai but occasionally visits San Diego to enjoy the blue skies and sandy beaches.

Andrew Thompson, Co-Founder, President & CEO, Proteus Digital Health
Andrew Thompson is Co-Founder, President and CEO of Proteus Digital Health. His vision for digital medicines is focused on expanding global access to care, dramatically increasing the value delivered by drugs and creating a more sustainable model for innovation that leverages the cell phone in everyone's pocket.
He is also a Co-Founder and Board Member of Summit Schools, a leading Charter School organization with an acclaimed track record and unique digital platform, featured in the Davis Guggenheim movie “Waiting for Superman”.Thompson is active in digital humanities innovation as a Member of the Stanford University Libraries and Academic Information Resources Council and with Cambridge University. He is a Co-Founder of Parker Library Online - a leading destination for digital medieval studies.
He holds master’s degrees in Engineering (Cambridge), Education (Stanford) and Business (Stanford GSB) and has a successful 25 year track record starting and building technology based healthcare companies in Silicon Valley.

Janet Xiao, Co-Chair of Global Life Sciences Group, Morrison & Foerster LLP
Janet Xiao, Ph.D., J.D., is co-chair of the global Life Sciences Group of Morrison & Foerster LLP and specializes in world-wide patent procurement, patent portfolio management, and strategic planning for life sciences companies. Recognized for her deep understanding of the pharmaceutical and biotech industries and knowledge of IP relating to the life sciences arena (Chambers Global), Janet is highly sought after for complex patent strategic and IP due diligence work. She is instrumental on the strategy for multibillion-dollar patent portfolios for pharmaceutical clients, and works extensively with U.S. and foreign life sciences clients in conducting IP due diligence in various business settings. Dr. Xiao is among the very few IP attorneys who are both equipped with solid skills in global strategic IP management and knowledgeable about IP issue unique to China and Chinese clients, and is frequently called upon to advise clients on IP issues and conduct IP due diligence in U.S./China cross-border transactions and operations in China. In 2014, 2015 and 2016, Dr. Xiao was listed multiple times in Chambers Global as a leading IP expert in the United States and a leading IP lawyer in China as an expert based abroad.

Michael Chang, M.B.A., Managing Director, Vivo Capital
Michael Chang joined Vivo with a background in healthcare strategy and operations in both the U.S. and China. Prior to Vivo, he worked at Johnson & Johnson, where he helped establish the strategy and commercial planning function for J&J’s China medical device business. Previously, he worked for Booz & Company and Fletcher Spaght, and also co-founded a company in the vascular access space.
Michael graduated from Harvard University with a B.A. in Economics, with a focus on health policy, and an M.B.A. from Harvard Business School. He also has research experience at Harvard Medical School and University of California Irvine in brain tumor immunology and cancer genetics.

Leidi Zhang, Managing Director, China Life Private Equity Investment Limited
Ms. Zhang is a Managing Director at the China Life Private Equity Limited. Founded in 2016, China Life Private Equity Limited, is the wholly-owned subsidiary of China Life Investment Holding Company Limited, which is the alternative investment arm of China Life Group. It currently manages the China Life Healthcare Equity Investment Fund of more than RMB 12 billion.
Ms. Zhang has over 13 years of experience in private equity investment and management consulting. Previously, Ms Zhang was an Executive Director in the Private Equity Department of China Life Investment Holding Limited. Prior to that, she worked for Roland Berger Strategy Consultants as a project manager. Ms Zhang graduated from Peking University with a Master Degree of Finance.

Wei Zhou, President & CEO, Centrillion Bio
Wei Zhou, Ph.D., J.D., is Founder, President and CEO of Centrillion. While a student at Stanford, he joined the pioneering genomic technology start-up Affymetrix (now NASDAQ: AFFX) where a generation of future industry leaders and scientists were inspired to change the world for the better through genomics. He continues to be fascinated by the profound and positive impact of genomic technologies in scientific research, health care, agriculture and other fields despite the severe limitations of current genomic technologies and is passionate about making genomic technology accurate and accessible.
Prior to founding Centrillion, Dr. Zhou was a partner at the technology law firm Wilson Sonsini Goodrich & Rosati where he advised life sciences, clean tech and digital media companies and venture capital investors. He also served as a senior advisor to the global law firm Covington and Burling. At Affymetrix, he was a vice president of intellectual property and advanced technology until 2008. He was also credited with building Affymetrix’ rapidly growing China business.
Dr. Zhou received his PhD from Virginia Tech (John Lee Pratt Fellow), his postdoctoral training from Emory University Medical School and his JD from Stanford University Law School (Associate Editor, Stanford Law Review). He is also a graduate of Harvard Business School’s General Management Program.

Lianshan Zhang, Senior Vice President and Global R&D President, Jiangsu Hengrui Medicine
Dr. Lianshan Zhang holds the position of Senior Vice President and Global R&D President of Jiangsu Hengrui Medicine. Hengrui is widely recognized as the domestic leader in pharmaceutical R&D innovation in China. In 2010, Dr. Zhang joined Hengrui and takes the responsibility for R&D strategy and management of dozens of NMEs across the globe, and was rewarded 1,000 Talents Plan. Prior to Hengrui, he was a senior research executive at Marcadia Biotech. From 1998 to 2008, Dr. Zhang was engaged in the discovery and development of peptide/protein therapeutics for the treatment of diabetes and obesity and successfully delivered multiple clinical candidates.
Dr. Lianshan Zhang obtained his undergraduate degree in Medicinal Chemistry from China Pharmaceutical University and his Ph.D. (summa cum laude) in Organic Chemistry at the University of Tubingen. He conducted his post-doctoral research at University of Tubingen and Vanderbilt University.

Howard Liang, CFO and Chief Strategy Officer, BeiGene
Howard Liang is the Chief Financial Officer and Chief Strategy Officer of BeiGene. Dr. Liang has more than 20 years of combined experience on Wall Street as an analyst covering the biotechnology and pharmaceutical sectors and as a scientist in the biopharmaceutical industry. Prior to BeiGene, Dr. Liang spent 10 years at Leerink Partners, where he was a Managing Director and head of biotechnology equity research. Dr. Liang was given many recognitions by the industry, including Institutional Investor All-America Research Team, Biotechnology / Mid- & Small-Cap, 2015; Bloomberg Markets Top US Analysts in Biotechnology, 2012; Wall Street Journal “Best of the Street” in Biotechnology and Pharmaceuticals, 2010 and in Biotechnology, 2008. Dr. Liang has also held senior biotechnology analyst positions at A.G. Edwards and JMP Securities. He began his career on Wall Street at Prudential Securities, where he was an associate analyst covering major and specialty pharmaceuticals. Before joining Wall Street, Dr. Liang spent eight years at Abbott Laboratories, where he was a senior scientist and a member of one of the industry's leading structure-based drug discovery teams. During his career as a scientist, Dr. Liang authored a review and 13 papers including 6 in Nature, Science, and Proceedings of the National Academy of Sciences.
Dr. Liang obtained his MBA and Ph.D. in Biochemistry and Molecular Biology, both from the University of Chicago, and his B.S. in Chemistry from Peking University.
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105-2482
|
|||||||
|
Marines' Memorial Club & Hotel 609 Sutter St San Francisco, CA 94102
CTIC Capital Healthcare Investment Summit
CTIC Capital is committed to create valuable connections for US companies that are interested in raising capital and developing business relationships in China.
http://www.cticcapital.com/2017-healthcare-investment-summit/
1. Get ready to pitch
We will bring you Chinese investors (100+) who are actively searching for US investment and business collaboration opportunities.
2. Get ready to be inspired
Impressive line up of investors from US and China to share challenges and opportunities in areas such as: Precision Medicine, Early Drug Development, Medical Devices, Cross Border M&A, Fund Raising and more.
3. Get ready to connect
Mobile APP would assist you in submitting inquiries of one-on-one investors meetings before and during the conference.
CABS member will get $200 discount (from $499 to $299) for tickets sold with 2017CABSCTIC discount code. Hurry, only 30 discounted tickets are available.
To register, click here.
SFO Marriott Waterfront, Burlingame, CA
 Happy Holidays from All of Us at CABS!
Happy Holidays from All of Us at CABS!
Thank you so much for being part of the CABS family! We would like to take this opportunity to express our gratitude for your active participation and strong support to CABS. We celebrated the unprecedented breakthroughs in Life Sciences and Medicine at our 2016 BioPacific Conference (See highlights) and we hosted more than 30 events together with our alliance partners this year.
CAB is a volunteer-based organization and we do not have any paid staff. We are extremely grateful to the generous support and commitment of our volunteers. In this new term, we have the largest Executive Council (EC) in the history of CABS. Sixty-one team members have dedicated themselves to better serve you and we have a very exciting line-up of events for 2017. We are starting the New Year with CABS Investor Forum on Jan 11 (Wed) and celebrating the Chinese New Year on Jan 29 (Sun). Our marquee event, 2017 BioPacific Conference will be held on June 24 (Sat). (See upcoming events for more details).
As 2016 draws to a close, we sincerely hope that you would kindly consider making a donation to CABS. We are a qualified 510(c)(3) non-profit organization and your charitable contribution may be tax deductible (Please donate). Furthermore, please also consider leveraging matching funds programs if available from your company to "stretch" your personal donation. Thank you so much for your kind generosity and we really appreciate your strong support to CABS!
Wishing you a wonderful holiday season and we look forward seeing you at our events in 2017!

Online
MIT Club of Northern California Seminar
Cancer Prevention & Anti-Aging through Micronutrients

December 8, 2016 | 6:00pm - 9:00pm
Morgan, Lewis & Bockius LLP
1400 Page Mill Road, Palo Alto, CA
CABS Member get $10 off using code "LSF1208". Register Here
Can a poor diet be just as bad as smoking cigarettes for your health? What can we do to protect our DNA and minimize the effects of aging and chronic disease?
Professor Bruce Ames, from University of California, Berkeley, the inventor of the “Ames test” —a simple assay for detecting DNA mutagens, knows a thing or two about carcinogens. He helped establish the first evidence that carcinogens mutate DNA and then helped create a catalog of mutagens, including cigarette smoke, permanent hair dye (aromatic amines), and flame retardant used in children’s pajamas (tris-BP).
Professor Ames developed a theory that DNA damage and late onset disease are a consequence of a"triage mechanism" developed during evolution to cope with periods of micronutrient shortage. Hear about the discoveries supporting his hypothesis which explains why diseases associated with aging, cancer, heart disease, and dementia, may be unintended consequences developed during evolution to protect us from immediate harm.
Join us on December 8, 2016 to learn more!
Contact Information
Primary Contact
Sooji Rugh
drleerugh@gmail.com
Morgan, Lewis & Bockius LLP; 1400 Page Mill Road, Palo Alto, CA
CABS DAY HIKE
San Bruno Mountain Park TRAIL
Sunday November 20th 9AM
Automatically cancel if rain probability >30%

HAT, SUNGLASS (AND/OR RAINCOAT)
WEAR COMFORTABLE WALKING/HIKING SHOES
DAY PACK WITH WATER AND SNACK/LUNCH
Please plan on arranging your own transportation to and from the hike
6$ Parking fee / Carpool is encouraged
Meet at the parking area at 9am
Take 101
Follow Bayshore Blvd and Guadalupe Canyon Pkwy to the destination
Or you may use the following address
555 Guadalupe Canyon Parkway, Brisbane, CA 94005
CABS SOCIAL LIFE
Online Registration is Free and Strongly Encouraged
555 Guadalupe Canyon Parkway, Brisbane, CA 94005
2016-2017 CABS Entrepreneur Club – Enrollment is Now Open!
Dear CABS members,
It is my great pleasure to invite you to join the 2016-2017 CABS Entrepreneur Club (CABS E-Club). The mission of this newly created E-Club is to foster entrepreneurship among our members. We aim to create an exclusive forum for our entrepreneurial members to discuss and incubate new business ideas, to find start-up partners, and to learn from experienced entrepreneurs and investors on how to build a successful business.
The key benefits for joining our E-Club include:
● Exclusive networking platform with life science entrepreneurs and investors
● Year-round small-circle activities and programs tailored to the needs of E-Club members. Some example activities include small-table private lunch with successful entrepreneurs or investors, mock-up business plan pitch day, new business set-up 101 training session, etc.
● Participate year-round in entrepreneur-related activities at CABS for free
We very much welcome active, entrepreneurial-minded members of CABS to join the E-Club. Enrollment is now open and we will close the enrollment process by the end of March 2017 or once we reach 50 members. The E-Club membership fee is $50 per year, separate from the CABS membership fee.
Please enroll by clicking the "Registration" button at upper corner or below (CABS membership is required to register; non-member may join CABS membership first)
We look forward to your joining the CABS E-Club family!
Sincerely yours,
Alexis Ji, Ph.D., MBA
CABS 2016-2017 Chair, Entrepreneur Club
Online Enrollment
|
Grand Copthorne Waterfront Hotel, Singapore
Minisymposium
Biomarkers for Immuno-Oncology:
Fundamentals, Technologies and Applications for Biomarkers in Research, Translational and Clinical Development of Immuno-Oncology Therapeutics
Speakers: Terri McClanahan (Merck), Rod Prell (Genentech), Michael Angelo (Stanford), Luciana Molinero (Genentech), Manasi Shah (Second Genome), Adil Daud (UCSF), Jeff Wallin (Genentech), Jakob Dupont (OncoMed Pharmaceuticals), Selda Samakoglu (Pharmacyclics)
Date: 10/28/2016
Time: 8:30-17:30 (continental breakfast starts at 8:00am; Meeting starts at 8:45am)
Registration fee (USD): Regular: $195; Between Jobs: $50; Academic: $50
Location: Crowne Plaza, Foster City, CA
Registration: http://www.pbss.org/aspx/eventInfo.aspx?eID=512
Registration deadline:10/26/2016 (it will close sooner if the seating cap is reached)
Crowne Plaza, Foster City, CA
Dear CABS members and friends,
It is my great honor to serve as President of this new term. Alex Zhang is the new President-Elect and Chair of the 2017 BioPacific Conference.
This term’s mission is focused on enhancing the scientific, business and social networking opportunities for our members. We are also broadening our vision to serve as the gateway linking life sciences professionals and organizations in the U.S. and Pacific Rim countries.
Please allow me to introduce you to the Executive Council (EC) of this new term. Thanks to the generous volunteer support of our members, we have the largest EC team in the history of CABS. Sixty-one team members have dedicated themselves to serve you in this new term.

Our Science Committee led by Wenfeng Xu and Ziyang Zhong will continue organizing workshops focused on cutting edge life sciences. We will be organizing a series of exciting seminars and workshops to showcase the latest trends.
Our International Collaboration (ICC) committee co-chaired by Xiaoli Qin and Wei Huang will be establishing new initiatives to facilitate talent recruitment, financing, entrepreneurship, cross boarder training and strategic collaborations between our members and companies/organizations in Pacific Rim countries. Simon Cen serves in the newly created Chapter Chair for China & Singapore position.
The Business & Career Development (BCD) committee is headed by Shenshen Hu and Dannis Chang. We will be expanding the reach of our highly popular Career Advisory Network (CAN) with mentors/mentees from partner organizations and organizing a series of career development workshops. We have just launched this term’s CABS Entrepreneur Club (CABS E-Club) and Alexis Ji is joining us in the newly established function of the Chair of the CABS E-Club.
Our Public Relations & Communications (formerly known as Publication) committee (PRC) is managed by Yang Tian and Susanna Chau. The team will strive to expand our social media presence on WeChat and help improve our communications with our members.
Liping Meng and Yonghua Pan will continue to serve as the co-chairs for our Membership committee this term. We will continue our membership outreach programs and look to expand the number of our volunteers.
Alliance Management (formerly known as Fundraising) committee is directed by Gavin Lu and Yan Wang. We will strive to improve our relationship with existing sponsors and to establish new strategic sponsorships.
CABS is never all work and no play. Our Social Life committee remains under the leadership of Ken Zhang and Sihong Zhou. We will be hosting traditional favorites such as hiking trips, workshops and parties.
Carrie Wang will continue to serve as the Chair of Operations (O2) and directs the O2 team to support all logistics for the EC. Our Webmaster is Michael Lin, and the Finance department is managed by David Wang and Yao Long.
On behalf of the 2016-2017 EC team, we hope to see you soon in person at our coming events and thank you so much for your strong support!
Sincerely yours,
Cheni Kwok, Ph.D., CLP
CABS 2016-2017 President
Email: cheni.kwok@cabsweb.orghttp://www.cabsweb.org/organization/
We will meet at the South Parking Lot of the Purisima Creek Trail on Highway 35 (Skyline Blvd). Address: South Parking Lot, Purisima Creek Trail, Redwood City, CA 94062.
Give yourself plenty of time October 16th is Pumpkin Festival in Half Moon Bay. Expect Traffic on Highway 92.

Online Registration is Free and Strongly Encouraged
South Parking Lot, Purisima Creek Trail, Redwood City, CA 94062

About CABS E-Club:
Aligning with our mission of catalyzing the professional interactions of our members in the life sciences industry, CABS is very pleased to launch our CABS Entrepreneur Club (CABS E-Club 2016-2017) to foster the innovative entrepreneurial spirit.
We are very honored to announced that Dr. Alexis Ji, Partner at Illumina Ventures, is joining us as our new Chair of CABS E-Club (2016-2017). The CABS E-Club is jointly hosted by the International Collaboration Committee (ICC) and Business & Career Development (BCD) Executive Councils. Please do join us to meet the team and to learn more about our plans for this new term !
Inaugural CABS E-Club Workshop:
Important Factors for Successful Career and Entrepreneurship in Biotech - The view from a former executive and scientist in pharma/biotech, and current venture capitalist
As CABS E-Club (2016-2017) inaugural event, we are very privileged to invite Dr. Jingshan “Jennifer” Hu, Partner at Qiming U.S. Healthcare Ventures. Dr. Hu will discuss and share her point of view of the important factors for success in career and biotech entrepreneurship. In addition, she will share her investment interests in therapeutics and healthcare technologies.
Discussion topics shall include:
- How to position yourself in a career path optimized for you?
- How can we as adult Chinese immigrants improve our communication skills and adjust to American culture?
- What are the differences between working for small biotech companies versus large corporations?
- Leadership versus management skills
- What are the important factors for biotech companies to attract VC investment and pharma partnering/licensing/M&A?
- What are important qualities of entrepreneurs that attract VC investment?
Agenda:
1:30 - 1:45pm Introduction to CABS & CABS E-Club
1:45 - 2:45pm Presentation by Dr. Jingshan “Jennifer” Hu
2:45 - 3:30pm Q & A and Networking
Our Speaker:
Dr. Jing-Shan “Jennifer” Hu, Ph.D., Partner Qiming U.S. Healthcare Ventures

Speaker Bio:
Dr. Jing-Shan “Jennifer” Hu is currently Partner of Qiming US healthcare Ventures. She brings extensive experience in partnering, licensing, R&D and management from three pharmaceutical multinational companies & two successful start-up and young biotech companies in the U.S. Prior to Qiming, she was VP of Bayer Healthcare and Head of its (External) Innovation Center China, responsible for partnerships for Global Drug Discovery with biotech and academia in Greater China. Before that, she was Director of Licensing & External Research at Merck & Co (MSD)’s for its worldwide licensing and partnering efforts on novel drug candidates and technologies from/with Greater China. She worked previously at Roche Palo Alto as Head of Functional Biology, at Affymetrix as Program Manager of Pharmacogenomics, and at Human Genome Sciences as Scientist of Protein Therapeutics & VEGF-2 Project Leader. She has been successful in partnering with at least 25 biotech and top academic institutions in both the U.S. and China. Dr. Hu obtained her post-doctoral training at Harvard Medical School, PhD degree from Univ. of Texas Graduate School of Biomedical Sciences at MD Anderson Cancer Center, and BS degree in Biochemistry from Peking Univ. She was instrumental at leading a successful and peaceful transition of the community of SAPA-West to Chinese-American BioPharmaceutical Society (CABS) as the President of 2006-2007.
Online Registration is free for all CABS members and required; Onsite registration is $10 per person.
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Dear CBA Alliance Members,
You are invited to attend the 2016 CBA Annual Conference. The theme of the conference is “Harnessing therapeutic development for sustainable growth”. We will explore many cutting-edge topics across the life sciences field through four sessions in this conference, which will provide a good platform for insightful discussions and stimulate new ideas. The conference will also provide excellent networking opportunities and enhance career development for attendees from academia and industry.
Please see below for more information of the annual conference. We strongly encourage you to register the conference online to take the advantage of the discounted price with lunch included. Please note, lunch cannot be guaranteed for onsite registrations.
CABS member will receive the discount, please use this link: http://www.cbasf.org/cba-alliance-registration/
(1) Early online registration (by Sep. 18th, 2016)
– CBA member $10
– Non-CBA member $35 ($10 off for alliance members)
(2) Regular online registration (by Oct. 4th, 2016)
– CBA member $20
– Non-CBA member $45 ($10 off for alliance members)
(3) Onsite registration
– CBA member $30
– Non-CBA member $55
Crowne Plaza, Foster City, CA

Simcere: Pioneer the healthcare frontier
Simcere is an integrated pharmaceutical group with leading development and commercial capabilities in China. Simcere is an emerging leader in R&D of innovative medicines and branded generics in China focusing on oncology, neurology, cardiovascular & metabolic diseases, Inflammation and Infectious diseases. In 2015, Simcere’s translational medicine and innovative drug R&D platform has been recognized as a State Key Laboratory by the National Ministry of Science and Technology. Simcere has also been actively pursuing cross-border partnerships to bringing global innovation and brands to China. Simcere has been nominated as top 100 in China pharmaceutical industry and top 10 most innovative companies in China for many years. Simcere of America has set up two offices, in Boston——international business development, New Jersey——international pharmaceutical business.

BioSciKin: Help more people with precision medicine
BioSciKin is a China-based biotechnology company focusing on precision medicine, drug innovation, incubation, and investment. It is recognized by State Ministry of China as a National Start-up Space. BSK is developing innovative precision medicine products, and is looking for partnership from all over the world for investment and co-development opportunities. We have formed strong alliances with multiple global ventures and invested over 20 start-up companies world-wide.
Looking into the future, our aim is to build:
•An open investment and incubation platform of precision medicine
•A leading precision medicine clinical test laboratory
•A pioneer in innovative drug development and commercialization
Click below for open positions:
Simcere will have four delegates led by their CSO attending CBA annual conference and hope to arrange 1:1 meetings with potential candidates.
Date: October 8, 2016 @ 8:30 am – @ 4:30 pm
Location: Crowne Plaza, Foster City, CA
For those who don't attend CBA annual conference, please contact Dongguowei@simcere.com directly to arrange 1:1 meeting on 10/8 at Foster City
Contact us: e-mail :dongguowei@simcere.comCompany and contact Wechat



Crowne Plaza, Foster City, CA
海纳英才 创业浦东
-- 2016海外人才创业上海自贸区行
为建设具有全球影响力的上海浦东科技创新中心,上海市浦东新区科技创新促进中心协同浙江清华长三角研究院共同举办2016海外人才创业自贸区行,旨在为 各国怀揣梦想的创业人才搭建创新创业平台。同时,本次活动将以创业大赛形式评选出优胜项目,可获得相应现金奖励及当地创业支持(免费入驻海外人才离岸基地并享受相关落地补助政策等)。
邀请对象:
国外:寻求创业平台依托,拥有激情和梦想的创业团队,重点关注电子信息、医疗健康等产业。
国内:境内外知名天使投资、VC/PE机构、海外人才服务机构、基地海外合作机构、加盟空间、孵化器及园区代表;
活动信息:
时间:2016年9月26至28日(25日报到)
地点:上海浦东
内容: “海纳英才·创业浦东”高峰论坛
“海纳英才·创业浦东”创业大赛
“海外人才创业交流分享会”及获奖项目颁奖仪式
活动期间食宿费用由主办方承担,国际旅费补助另行通知。
报名方式:
请于2016年8月31日前填写附件报名表,并发送至info@tsinghuadelta.com ,报名截至日期后一周将收到回复。
活动咨询:
吴 毓 琦(北美: +1-408-881-3298)
陆 云 亮(中国:+86-139-6736-7963,+86-573-83998576)
陈 佳 妮(中国:+86-021-58817125)
组织机构:
主办单位:上海市科学技术协会
上海市浦东新区区委组织部
上海市浦东新区科委科协
上海张江高科技园区管理委员会
承办单位:上海市浦东新区科技创新促进中心
协办单位:上海普德科技发展有限公司
浙江清华长三角研究院
支持单位:中国科学技术协会海智计划办公室
中国(上海)自贸区海外人才离岸创新创业基地 简介:
中国(上海)自由贸易试验区海外人才离岸创新创业基地是由上海市科协、中国(上海)自贸试验区管委会、浦东新区等共同建设,面向海外人才,区内注册、海内外经营。
基地定位:以低成本、便利化、全要素、开放式、配套成熟完善的空间载体为基础构建,具备引才引智、创业孵化、专业服务保障等功能的国际化综合性创业平台。
主要功能:一是引进和集聚海外高端人才,支持海外人才开展离岸研发、离岸贸易和离岸金融等业务;二是探索离岸创业模式,促进创新项目海内外预孵化和成果转化;三是开发和培育专业服务能力,通过模式创新和政策支持,促进海外优质创新资源集聚。
资源优势:物理空间多点布局,首批实体平台设在自贸试验区张江片区、陆家嘴片区、保税区片区和临港片区;依托科协系统“海智计划”联系网络,建立与国外机构团体的合作,集聚海外人才资源;结合自贸试验区制度创新,探索海外高层次人才创业项目在离岸企业注册、离岸业务税收、高新技术企业认定、知识产权认定和上海市审批等方面的制度创新。
浙江清华长三角研究院 简介:
浙江清华长三角研究院由浙江省人民政府与清华大学联合组建,是综合性高科技研发、项目孵化、科技合作、投资交流平台。
Ø创新平台包括生物技术与医药、生态环境、先进制造技术、信息技术研究所、现代农业等5个研究所,2个国家级研究中心,1个国家级产业技术联盟,2个省级重点实验室和40多个企业联合研发中心。
Ø在美国、德国、英国、以色列、日本、加拿大、芬兰等全球人才腹地建立起国际孵化器,打造“跨越太平洋的人才直通车”。
Ø探索建立包括天使基金、风投基金和股权私募基金在内的多层次科技投融资体系,累计对外投资60余家中小型科技企业,其中4家企业成功上市。2015年新成立研究院浙华紫旌母基金,首期规模10个亿,投资11支子基金,涉及6大领域,总规模70亿,实现科技与金融的有机结合。
xxx
上海浦东
The Redefining Early Stage Investments (RESI) Conference is an ongoing conference series that will be establishing a global circuit for early stage life sciences companies to source investors, create relationships, and eventually, get funding. The RESI conference focuses on the diverse breadth of early stage investors that LSN tracks, including Family Offices, Venture Philanthropy Funds, VCs, Angel Groups, Corporate Venture Capital Funds, and more. The RESI Partnering Forum allows fundraising executives to identify and book up to 16 meetings with life science investors who fit their company’s technology sector and stage of development. Additionally, through an expansive series of panels and workshops, attendees will have the chance to hear firsthand accounts from investors explaining their current investment mandates and process for identifying and qualifying candidates.
Click Here for more details and registeration
The Westin Copley Place, 10 Huntington Ave, Boston, MA 02116
Please register online. If there are any questions, please email icc@cabsweb.org
Agenda:
6:30-7:00pm networking
7:00-8:00pm presentation by Dr. Jiang Feng
8:00-9:00pm Q&A
9:00-9:30pm networking
The topics:
Ⅰ.Overview of China Medical Devices and Materials Industry
Ⅱ.Main Industrial Policies in Recent 3 Years
Ⅲ.Impact of Above Policies on China medical device and materials Industries
Ⅳ.Effect of Above Policies on "Returnees Projects "
Ⅴ.Main Points for Successful Launch of "Returnees Projects "
1.Selection of projects appropriate for China
2.Selection of correct business model for China market
3.Confirmation of the landing city (industrial park)
4.Determination of partners
5.Financing and government fund utilizing
6.Cases analysis
Ⅶ.Analysis of Cooperation Opportunities in Medical Devices and Materials Industries Between US and China
1.Differences and complementation of US -China market: status and evolvement
2.US -China cooperation: new business model in China
3.Analysis of American local business pattern for Sino-American cooperation
4.Prospects of US -China cooperation
Ⅷ.Brief Introduction of CAMDI, associations, and innovation service platform
演讲主题:
一、中国医疗器械及材料产业概况
二、近三年的主要产业政策
三、当前政策对中国本土产业的影响
四、当前政策对海归项目的作用
五、海归项目落地成功要点
1.选择适合中国的项目,
2.选对进入中国的业务模式,
3.海归项目落地城市(园区)的确定,
4.合作伙伴的选择,
5.如何融资及利用政府资金
6.案例分析
六、中美医械材料产业合作机遇分析
1.中美市场差异及互补:现状和演变
2.中美合作的中国业务热点及趋势
3.中美合作的美国本土业务模式分析
4.中美产业合作的前景展望
七、协会及国家创新服务平台简介。
The speaker:
Dr. Jiang Feng received his M.D and PhD in Clinical Medicine in 1995, and got EMBA in 2006 ;Jiang is currently appointed the executive chairman and CEO of China Association for Medical Devices Industry (CAMDI), Chairman of China Strategic Alliance of Medical Device Innovation, Deputy Director of China National Biomedical Engineering Education Supervisory Committee. Dr. Jiang is also the standing director at many associations and guest professors at a number of universities. Jiang is also an expert consultant for the Ministry of Health, CFDA,Ministry of Science and Technology(MOST), Ministry of Industry and Information Technology, and a number of national and local medical science parks.
After working in the hospital as a thoracic surgeon for 12 years, Jiang founded Shaanxi Huaxin Pharmaceutical Co. in 1997 which focused on drug research and distribution. Huaxin was later sold to a listed company after three years of rapid growth, its R&D product made huge profits in the following years. Because Huaxin's success, Jiang was invited to work for Sinopharm , the largest national medical devices and pharmaceutical group. The following 10 years saw him bringing considerable growth to the business. During his 7 years’ term as the general manager at China National Medical Equipment cooperation(CMIC), Jiang established the largest medical device distribution system in China, with annual revenue exceeding 1 billion RMB (2008). He successfully restructured CMIC, transforming the company from an exhibition-focused business to a large scale production and marketing enterprise. Jiang had served as the chairman of China Association for Medical Devices Industry (CAMDI) for 8 years, during which he participated in many policy-making processes that impacted the whole industry. While in CAMDI, Jiang visited over a thousand members and provided services for many of them, directly leading to CAMDI's rapid growth to more than 3000 members. In 2009, Jiang left CMIC to become the Chairman of China Strategic Alliance of Medical Device Innovation, an organization initiated and established by MOST. Today, the alliance has assisted MOST to finance and manage hundreds of medical devices innovation projects, the total budget of which amounts to over 2 billion RMB.
Most recently, Jiang has focused on financing and other services for innovative medical devices projects, Working with his team, Jiang has already helped many domestic and overseas startup projects successfully land in science parks by connecting them with capital and partners In China, Jiang has set up several one-stop service centers in some top science parks for innovative medical technologies; Jiang serves as independent director and expert consultant in many listed companies and VC. With 30 years of experience in the hospital and industry, Jiang is familiar with the strategic operation of pharmaceutical and medical device companies, and has established strong, mutually trusting relationships with many manufacturers, distributors, investment institutions, R&D institutions and government agencies.
Dr. Jiang hopes to continually serve more medical innovative cooperation and business between China and US.
姜峰,第四军医大学临床医学博士,清华大学EMBA, 曾作为胸科医生在医院工作12年,于1997年离职创立了陕西华信医药有限公司开展药物研发及分销,公司业绩增长迅速并于三年后成功转让给某上市公司;此后姜峰受聘于央企国药集团长期担任国家级大型医药和器械公司总经理,并使相关公司业绩迅速提升;在中国医疗器械公司任职期间创建了境内最大的器械分销系统 并完成了公司改制和从会展业务向产销企业的顺利转型;姜峰任协会会长及常务副会长已13年,期间参与制修订多项行业政策法规,走访过千余家会员企业并为其 提供各类服务,使协会不断发展壮大受到业内一致好评;2009年底姜峰辞去企业职务出任科技部国家医疗器械产业技术创新联盟理事长,至今联盟已协助科技部 对数百个医械创新项目进行资助及后续管理,涉及经费逾20亿元;近年来姜峰在资本与创新对接及科技成果转化领域倾注较多精力,率领团队促成多个境内外创新 项目与资本对接后落户科技园区,并在多个政府部门、科技园区、境内外上市公司及风投机构任专家顾问、独立董事及合伙人;姜峰还作为产业界唯一代表出任教育 部生物医学工程教指委副主任已10余年,在促进产学研合作方面成绩显著,多年的业内工作使姜峰熟悉医药及器械企业运作管理,与众多国内外生产企业、经销 商、科研机构、投资机构、协会、政府和科技园区建立了良好关系,姜峰愿为中美医学创新合作提供更多的专业服务。
Hanqi Investment, 1633 Bayshore Hwy, Unit 280, Burlingame, CA 94010
Advanced Cell Diagnostics, Inc. (ACD) is a leader in the emerging field of molecular pathology, developing cell- and tissue-based diagnostic tests for personalized medicine. The recent ACD acquisition by Bio-Techne (here) is probably the largest M&A for a biotech tool company founded by a Chinese American scientist in the US biotech area. ACD's proprietary RNAscope® Technology is a multiplex fluorescent and chromogenic in situ hybridization platform that is capable of detecting and quantifying RNA biomarkers in situ at single molecule sensitivity. In this special workshop, we will hear from the founder and CEO of ACD, Dr. Yuling Luo, and Dr. Ken Fong from Kenson Venture, who has helped shepherd ACD from its inception to acquisition.
Presentation: my learnings from three startups
Yuling Luo, PhD
Founder, President, and Chief Executive Officer, Advanced Cell Diagnostics
Dr. Luo is an entrepreneur with more than 14 years of start-up experience in leadership and innovation. Before ACD, Dr. Luo was a founder of Panomics (previously named Genospectra before its acquisition of Panomics), a life science company that was acquired by Affymetrix in 2008. At Panomics, Dr. Luo served various roles including Vice President, Functional Genomics and Chief Scientific Officer and he led the development and launch of all of its flagship product lines. Prior to Panomics, Dr. Luo was one of the earliest senior scientists at Exelixis, where he led a team to identify and validate novel therapeutic targets. Dr. Luo has published many research papers in top peer-reviewed journals. His inventions are the subject of over a dozen patents and patent pending applications. Dr. Luo received his doctorate in biochemistry from Case Western Reserve University and conducted post-doctoral research at the University of Pennsylvania.
Kenneth Fong, PhD
Founder and Chairman of Kenson Ventures, LLC
Dr. Fong sits on the board of five biotech companies ranging from Innovative tool and diagnostics to pharmaceutical development. In his 30 plus years in the biotech business, he is well-respected in the industry for cultivating and mentoring a number of successful entrepreneurs and companies.
Dr. Fong is currently the founder and chairman of Kenson Ventures, LLC, a company that specializes in crafting development strategy for early-stage and mid-stage biotech companies. With Dr. Fong at the helm, Kenson has promoted and facilitated the acquisition of a number of its PORTFOLIO companies, including Advanced Cell Diagnostics (acquired by BioTechne), SA Biosciences (by Qiagen), DHI (by Quidel), Fermenta (by ThermoFisher), Panomics (by Affymetrix), Epitomics (by Abcam), and has helped two others, Bioform and Optimer, go public. Along with cultivating entrepreneurs at Kenson Ventures, Dr. Fong is leading System Biosciences, a biotech company that he also founded.
An experienced entrepreneur even before Kenson, Dr. Fong founded and served as CEO of Clontech Laboratories from 1984 until the company was acquired by Becton Dickinson in 1999. Clontech, a leader in the molecular/cell biology market, was the largest company of its kind founded by an Asian American. It was recognized as one of the fastest-growing companies in the Bay Area in 1994, 1995, 1998 and 1999.
In addition to running several companies, Dr. Fong has held a number of other leadership positions over the years. He was president of the Bay Area Asian American Manufacturers Association in 1987 and of the Society of Chinese Bioscientists in North America from 2007 to 2009. Dr. Fong has also been a member of the Committee of 100 and served on several boards including the Board of Trustees of California State University, the Advisory Board of the College of Science and Engineering at San Francisco State, and the Board of Associates at the Whitehead Biomedical Institute at MIT.
Dr. Fong has a number of philanthropic interests. He was one of the lead supporters of the Tech Museum of Innovation in San Jose, the Chinese Historical Society in San Francisco, the bioengineering auditorium at UC San Diego, and graduate seminar programs at Indiana University. He has also provided a number of scholarships to San Francisco State University, Peking University and the California State University system. Dr. Fong was involved in establishing the Fong Optometry and Medical Library at UC Berkeley, the Kenneth Fong Endowed professorship in Bioengineering at Stanford and the Translational Science Awards at San Francisco State University.
Dr. Fong received his B.S. from San Francisco State University and his Ph.D. from Indiana University.
Morrison Foerster office in Palo Alto (755 Page Mill Road, Palo Alto, CA)
InnoBay 宁波杭州湾新区国际创新创业大赛
For more details, please click here
If you are interested in registering for the event, please contact: fundraising@cabsweb.org
China
Dear CABS members,
I am so pleased to inform you that we have received a total of 48 applications to join the CABS Executive Council (EC) for this new term ! Thank you so much for your strong support and participation. Together, we can launch new initiatives and events to better serve our members and to strengthen our CABS brand.
Now is the time for you to elect your EC. All CABS members are entitled to vote. You can view the profiles of our applicants here. Please kindly login to our website to cast your vote of support for the new EC.
Voting will close on September 9 (Friday) at 9PM PT.
We will introduce you to the new EC and highlight our plans for the new term in October. Please stay tuned !
Thank you very much for voting and we truly appreciate all your support !
Cheni Kwok Ph.D., CLP
CABS President-Elect
Email: cheni.kwok@cabsweb.org
http://www.cabsweb.org/ec-election-2016/
Please register for free to participate in this event. Please email icc@cabsweb.org for any questions.
Nanjing Sanhome Pharmaceutical Co., Ltd. Business Development & Recruiting Event in San Francisco
The chairman & CEO of Nanjing Sanhome Board, Dr. Yong Wang, with a group of 20 delegates from the Sanhome Board and Senior Management, will join a business development & recruiting event at 1:00 pm on Aug 20th at LKSC 120 Stanford University.
Dr. Wang will introduce Sanhome Pharmaceutical and share his 20-year experience in establishing a pharma company in China and how to bring Class I innovation drug to China market. Sanhome is:
- l Recruiting a CSO, who will be in charge of global R&D pipeline and overall operation, location: Nanjing
- l Recruiting principle and senior scientists in different departments, over 30 positions, location: Nanjing
- l Looking for merger and acquisition opportunities of therapeutic companies including preclinical and clinical trial projects and products
- l Looking for investment opportunity in biotech companies, including small molecule and large molecule therapeutics
- l Looking for other business partnering opportunities in new drug R&D to enrich the pipeline of Sanhome which has applied for IPO
Time & Location
- · 1:00 pm – 4:00 pm, Sunday, 20th August, 2016. Contact Phone: 317-504-6592.
- · LKSC 120, Stanford University, 291 Campus Drive, Palo Alto, CA94305.
Organisers
- · Nanjing Sanhome Pharmaceutical Co., Ltd. & Chinese American Bio/pharmaceutical Society.
- · Thanks for the support from Association of Chinese Students and Scholars at Stanford.
Schedule
- · 1:00 – 1:30, Sign in and network
- · 1:30 – 1:40, Introduction of CABS
- · 1:40 – 2:40, Introduction of Sanhome, by the president, Dr. Yong WANG
- · 2:40 – 3:00, Q & A
- · 3:00 – 4:00, Refreshment & reception
Introduction
- · Founded in 1996;
- · At the end of this year, total employees expect to reach 2,500, 10-15% are R&D staffs including 200 with Ph.D. or Master degrees;
- · R&D centre with over 8.6 acres, a pharmaceutical plant, a bulk pharmaceutical chemicals plant, a GAP level cultivation base, and with more than 400 branches or offices all over China;
- · GMP certificated, ‘State high-tech innovation enterprise’ by China MST, A top 100 pharmaceutical enterprise in China;
- · Have developed 1st class new drugs and exclusive products, and holds 8 overseas patents, 34 China patents and more than 100 new drugs certificates;
- · Undertakes ‘863’ and other national major projects, with a post-doctoral working station;
- · R&D budget 200 million CNY this year, which represents 9% of the total sales; will increase to no less than 15% and the total R&D budget will be over 1 billion CNY within 5 years.
Requirements
- · Chief Science Officer(CSO):Ph.D. with 5-10 years of experiences in pharmaceutical industries;
Salary & welfare
- · Benefits: free housing, stock, funding etc.
Contact:
- · Phone: 317-504-6592
- · Email: zhanghx@sanhome.com, oversea_recruit@sanhome.com
南京圣和药业2016年8月20日旧金山专场招商招聘会
南京圣和药业王勇董事长帅圣和董事会和高管团队一行20人, 将于8月20日下午1:00到斯坦福大学李嘉诚中心召开圣和药业专场招商招聘活动。王勇董事长将亲自介绍圣和药业的发展现况和愿景,以及20年来他在圣和创业的经历,包括成功开发一个国家一类新药。圣和药业是国内拥有一类新药成功开发上市的不多的药企之一。圣和的研发和销售团队,成功经历了临床,上市,医保,招标,进医院,推广等新药在中国上市的每一个环节,他们的经验分享,能帮我们了解中国新药开发的历程。
本次活动,圣和药业将:
- l 在全球高薪聘请CSO首席科学家,全面负责圣和研发管线,和研发策略战略。
- l 招聘高级研究员和资深研究员,R&D 各个职能部门,30个以上的职位
- l 寻找收购并购的机会,包括制药项目或者公司,临床前或临床阶段。
- l 寻找投资BIOTECH公司的机会,小分子和大分子制药项目。
- l 公司处于排队上市的高速发展期,寻找各种合作方式,共同开发创新药PIPELINE。
时间地点
- l 2016年8月20日星期六,下午1:00 – 4:00。联系电话:317-504-6592
- l 斯坦福大学李嘉诚中心LKSC120,291 Campus Drive, Palo Alto, CA 94305
承办单位
- l 南京圣和药业股份有限公司、中美生物医药协会 鸣谢:斯坦福中国学生学者联合会
活动流程
- · 1:00 – 1:30,报到登记
- · 1:30 – 1:40,中美生物医药协会介绍
- · 1:40 – 2:40,圣和药业介绍(董事长:王勇博士)
- · 2:40 – 3:00,提问环节
- · 3:00 – 4:00,交流活动,配有茶点
企业介绍
- · 始建于1996年;
- · 截至今年年底,员工总人数达2500人,研发人员占10-15%,其中博士、硕士200余人;
- · 研发中心占地2万平方米,公司另有中药材GAP种植基地、120亩原料药生产基地、100亩制剂厂、及遍布全国的400余个负责学术推广的办事处;
- · 第一批通过全国新版GMP认证,科技部“重点高新企业”、全国工商联评定“2015年中国医药制药业百强企业”;
- · 拥有国家一类新药、全国独家产品等,国外发明专利8项、国内发明专利34项、新药证书百余本;
- · 承担国家863项目、国家火炬计划等重点科研项目,拥有博士后工作站;
- · 今年研发预算2亿元,占总销售收入9%,五年后占比将不低于15%、研发投入绝对值将超10亿元。
- · 公司在证监会排队上市。
基本条件
- · 首席科学家CSO:博士学历,5-10年以上跨国或国外药企相关工作经验。
薪酬待遇
- · 首席科学家CSO, 工资面议。
- · 福利待遇:住房、股权、人才计划、餐饮等等。
联系方式:
- · 电话:317-504-6592
- · 邮箱: zhanghx@sanhome.com, oversea_recruit@sanhome.com
LKSC 120, Stanford University, 291 Campus Drive, Palo Alto, CA 94305
CABS Needs You :
CABS Executive Council
(2016-2017) Application is now Open !
Dear CABS members and friends,
CABS has been proudly serving 3000+ members, sponsors and friends of the life sciences communities in the U.S. Bay Area, China and Pacific Rim countries for the last 18 years. We organize year-round programs and workshops to showcase the latest advancements in life sciences, foster collaborations and entrepreneurships, facilitate mentorship and career development and create networking opportunities for our members.
We are now calling for candidates to run for our Executive Council (EC) for 2016-2017.
Our EC is the heart and soul of CABS, and orchestrates our activities throughout the year. In the fall of 2016, the new EC will be taking the leadership role in planning and organizing exciting new programs and initiatives both locally and abroad. This is your opportunity to lead and to make significant contributions to our organization. With your participation, we can elevate CABS to the next level!
To participate in our EC, please kindly submit your application here no later than 28 August 2016.
Very much look forward to working together and really appreciate your kind support !
Cheni Kwok, Ph.D., CLP
CABS President-Elect
Email: cheni.kwok@cabsweb.org
cabsweb.org
Life is short and time is long. After the year of the monkey and the month of the horse, we are finally approaching the day of fun: the CABs Summer BBQ!!!
CABS 2016 Summer BBQ
Date: August 6th, Saturday, 11 am-3 pm
Location: Boothbay Park, Foster City
You and your family are cordially invited to our Annual Summer Networking Picnics, hosted by CABS Social Life Committee.
BBQ grilled pork, beef, chicken, hot dog, hamburger, salad, soft drinks, fruits, desserts and a lot more!
All you can eat and drink! With a bonus of unlimited FUN and unexpected opportunity for making new friends and social/career interaction!!
Lucky draws: $100 gift card, $50 gift card and a number of other secret surprising!!!
Online registration is strongly suggested.
Online:
Active CABS member: $5
Non-member: $10
Senior >65 or Child <15: $1
Onsite:
Active CABS member: $10
Non-member: $15
Senior >65 or Child <15: $1
On-line Registration closes on August 5th, 12:00 PM (noon).
Boothbay Park, Foster City
Are you eager to learn the most advanced academic research in biomedical field? Do you want to disclose the secret of the most popular bio-device company in the Bay Area? Are you curious about how to commercialize the product from the lab bench? Chinese-American Biopharmacuetical Society with ACSSS-Postdoc Department brings you the workshop – The New Wave of Therapy – Organ Transplantation & Bio-device. Three top notch scientist, CEO, and entrepreneur will take you to a journey of tech and business brainstorming. June 18th at Stanford LKS center, we are waiting for you!
Keynote Speaker:
Prof. Hiromitsu Nakauchi, Stanford University
Title: “Organs niche” for Generation of Functional Organs from iPS Cells
Invited Speaker:
Ben Hwang, Chairman and CEO, Profusa. Inc.
Title: Know Thyself – How a Little Sensor That Works for More Than Two Years Can Change The
Way You Understand Your Body and Health
Andrew Lee, Co-Founder and Chief Scientific Officer, Stem Cell Theranostics
Date: 2016-06- 18 Saturday
Time: 12:30 pm to 4:30 pm
Location: Li Ka-Shing Center, LK130, Stanford, CA 94305
Registration Link: click here
Hiromitsu Nakauchi:
Prof. Nakauchi obtained a M.D. from Yokohama City University School of Medicine and a Ph.D.
in immunology from University of Tokyo Graduate School of Medicine. He isolated CD8 genes
during his post-doc period at the Laboratory of Prof. Leonard Herzenberg at Stanford
University. After returning to Japan, he started working on hematopoietic stem cells in his
laboratory at RIKEN. In 1994, he became Professor of Immunology at the University of Tsukuba
where he demonstrated that a single hematopoietic stem cell could reconstitute the entire
hematopoietic system, a definitive experimental proof for the “stemness”. Since April 2002, he
has been a Professor of Stem Cell Therapy in the Institute of Medical Science at The University
of Tokyo (IMSUT). In 2008, he was appointed Director of newly established Center for Stem Cell
Biology and Regenerative Medicine at IMSUT. Just recently, he returned to Stanford University
as a faculty to continue his stem cell research at the Institute of Stem Cell Biology and
Regenerative Medicine. Goals of his work are to translate discoveries in basic research into
practical medical applications.
Ben Hwang
Ben currently serves as the Chairman and Chief Executive Officer of Profusa, a company that
has created a wear-and- forget way to stream biochemical data from inside the body to a smart
device outside the body, truly transforming the way we measure health and wellness.
From his early exposure as an undergraduate research fellow at the lab of Leroy Hood at
Caltech, where the automated DNA sequencer was developed, to bringing cutting edge life
sciences tools to the market at Life Technologies Corporation (acquired by Thermo Fisher
Scientific, Inc.), Ben has seen first-hand the transformative impact that science and technology
have to change our world. Prior to Profusa, Ben served in a variety of leadership roles at Life
Technologies Corporation, including as President of the Asia Pacific Region and Head of the
qPCR Division. A former management consultant at McKinsey and Company, Ben earned his
M.A. and Ph.D. in Biology from the Johns Hopkins University.
Andrew Lee
Dr. Andrew Lee is a Co-Founder of Stem Cell Theranostics, Inc and was the company's founding
CEO before transitioning to Chief Scientific Officer. As an MD/PhD student at Stanford
University, Dr. Lee worked closely with Dr. Wu and Dr. Robbins, to develop the core disease
modeling technology that is the foundation of the company, publishing over 50 peer reviewed
research manuscripts on the use of stem cells for treatment of heart disease and drug
screening. Dr. Lee also the Co-Founder of StartX Med, the official Medical Innovation
Accelerator of Stanford University and Stanford Healthcare, which has 85+ portfolio companies
to date with cumulative funding of over $350M. His research has been supported by the
Howard Hughes Medical Institute, the FDA, and the American Heart Association as well as
several of the top 10 global pharmaceutical companies.
Hosted by: CABS and ACSSS-Postdoc Dept
Stanford student and postdoc or affiliates- FREE
CABS Member Access – FREE
Non Stanford/CABS Members – $20 (USD)
Registration Link: click here
Li Ka-Shing Center, LK130, Stanford, CA 94305
PATENT 101 seminar is postponed to September - stay tuned (this information is updated on June 1, 2016)
If you have any questions, please email icc@cabsweb.org
If you are interested in attending this seminar, please register online. Registration is free.
Meanwhile, when you register, please let us know the area(s) of patent law you are interested in learning more about as well as the specific question you may have so we can try to explain it during the seminar.
If you have trouble to register, please email icc@cabsweb.org
Agenda:
6:00-6:20 pm networking
6:20-7:00 pm presentation
7:00-8:30 pm questions and answers
8:30-9:00 pm networking
At this patent seminar, two patent attorneys, Dr. Lei Fang and Mr. Jigang Jin, will share with you what patent issues you may face and how to address these issues when you start your new biotech company.
They will use the hypothetical example below to introduce and discuss the following basic patent law and license issues in the bio-pharma life sciences fields:
- When and where should a patent application be filed?
- What can be patented (patentable subject matter & types of patent applications)?
- What are the basic criteria for granting a patent (patentability requirements)?
- What are the timeline and cost for obtaining a patent?
- Ownership vs. inventorship of a patent
- Technology transferring and licensing (exclusive vs. nonexclusive; terms, sublicense, payments, fields and territories of license, etc.)
Hypothetical example:
Dan is a scientist and entrepreneur, starting a new biotech company XYZ, Inc. which will develop certain therapeutics for cancer treatment. One of the technologies that will be used to develop those therapeutics is protected under a third party’s patents. To avoid potential infringement of these patents, what and how should Dan do before he can even use the third-party’s patented technology in the development of his own company’s therapeutics?
Also, Dan has conceived some ideas on molecular structures of certain lead products for the therapeutics he is going to develop, as well as certain unique methods of making and/or using these potential lead products for preventing and/or treating certain disease conditions. Some of these ideas have been tested experimentally in the laboratory animals and cell cultures. However, no actual clinical or human studies has been conducted at this point. In addition, more than one year ago, Dan has published a paper describing certain researches he had done which may lay down the groundwork for the development of these therapeutics. When, what, and how can Dan do to protect his own developments of the lead therapeutics, as well as the methods of making and/or using these therapeutics?
Presenters:
LEI FANG, Ph.D., Patent Attorney
Dr. Lei Fang advises domestic and international clients on a wide range of intellectual property matters. She helps clients identify key intellectual property and develop portfolio protection strategies. Lei prosecutes patent and trademark applications before the US Patent & Trademark Office, and counsels clients and provides legal opinions on patentability, validity, non-infringement, and freedom-to-operate issues primarily in the life sciences sector related to biotechnology, pharmaceuticals, and medical devices inventions. Lei also helps clients on technology transferring and licensing and FDA regulatory reviews for drugs and medical devices. She also conducts intellectual property due diligence and provides product clearance opinions and development strategies. Her experiences in patent litigation include participating and providing technical support in the Hatch-Waxman ANDA (Paragraph IV) patent litigation involving generic pharmaceuticals, and managing International Trade Commission Section 337 investigations and related district and appellate court cases involving Chinese companies. Lei also helps clients to obtain a copyright registration before the US Copyright Office.
For more than a decade, Lei has worked with clients ranging from multinational corporations, to early stage entrepreneurial companies, to nonprofit university and science research institutes. Her extensive knowledge on intellectual property related matters in the biotechnology, pharmaceutical, medical device, and life sciences industries have led to many speaking engagements for her.
With her native Chinese background, Lei speaks and writes fluent Chinese and offers clients a unique combination of legal knowledge, advanced scientific and technical training, and an intimate understanding of the Asian marketplace. Lei is actively involved in many Asian-American organizations, many of which she serves as a legal counsel. She also represents multiple Chinese companies in various corporate and intellectual property matters in the United States.
Before starting JIN & FANG LLP, Lei worked as a patent attorney at a few prestigious US firms (e.g., Sutherland Asbill & Brennan LLP and Troutman Sander LLP). Prior to launching her legal career, she had a postdoctoral training at Harvard Medical School/Brigham Women’s Hospital. She also worked as a research scientist at a biotech/pharmaceutical company in Cambridge, MA.
JIGANG JIN, Patent Attorney
Jigang’s practice focuses on patent prosecution, counseling, and litigation in industries including telecommunication, consumer electronics, semiconductor, computer software and hardware, wireless and mobile, Internet, robot, artificial intelligence, and medical device. His deep technical knowledge and experience allow him to quickly understand clients' technology and bring optimal legal solutions for client's issues.
Jigang has helped both well-established companies as well as startup companies obtain patents. In addition, he has litigated cases in U.S. district courts and participated in International Trade Commission investigations. Before starting JIN & FANG LLP, Jigang worked as a patent attorney at Ropes & Gray LLP, Skadden, Arps, Slate, Meagher & Flom LLP, Cooley LLP, Blakely Sokoloff Taylor & Zafman LLP.
Prior to attending law school, Jigang worked as a software engineer designing and developing distributed and web-based software systems for major insurance companies. He also has experience working on various software system integration projects.
1633 Bayshore Hwy, suite 280, Burlingame 94010
 PBSS Workshop: Pharmacology animal models in drug development: fundamentals, applications and case studies related to oncology, metabolic diseases, inflammation and immunology
PBSS Workshop: Pharmacology animal models in drug development: fundamentals, applications and case studies related to oncology, metabolic diseases, inflammation and immunology
About the Topic
Preclinical research and clinical research are two major components in the process of drug development. In preclinical research, one of the most important considerations is the use of animal models to study efficacy, mechanism of action and establish the pharmacokinetic/pharmacodynamic (PK/PD) relationship. Together with toxicology studies, these efficacy-related studies are essential for any new drug candidate to advance to clinical development and eventually reach the market. Questions are routinely asked and debated such as “how to select relevant animal models”, “how to optimize rodent models for reliability and predictability”, “what are the pros and cons of different models”, and “how to translate the animal results to clinical studies”. We have assembled an experienced team of speakers from academia, the biotech/pharma industry and contract research organizations to discuss these critical questions.
The workshop is designed for both pharmacologists and non-pharmacologists. Non-pharmacologists will benefit as they will gain a better technical understanding of the process and challenges, while experienced pharmacologists can see the best practices used by their colleagues at other companies.
The following topics will be covered at the workshop:
- Animal models in translational research: Fundamentals and Applications (Dirk Mendel, E-Scape Bio)
- Oncology: animal models and case studies (Stephen Gould, Genentech)
- Autoimmune Disorders/Inflammation: In vivo models and case studies (Rana Samadfam, Charles River Laboratories)
- Metabolic diseases: Fundamentals of Animal Models of Metabolic Disease (Peter Havel, UC Davis)
- Improving the predictability of rodent models (Leon Hall, Mousera)
Detailed Presentation Outlines:
Oncology (Dr. Dirk Mandel, E-Scape Bio and Dr. Stephen Gould, Genentech)
An overview of how to approach a new project from a translational perspective; how to start identifying the key questions for a given project
- A historical perspective to the use of preclinical mouse models in oncology research
- An understanding of the pros and cons of various preclinical oncology models and a working knowledge of how to select the most appropriate model or models
- Familiarity with best practices for the preclinical evaluation of oncology drugs in mouse models
- A full understanding of PK/PD/efficacy relationships and how to apply these to set robust clinical gates
- An understanding of exposure-response relationships and how these may impact decision making in the clinic
- An appreciation of how to approach multi-targeted vs specific compounds
- How to use reverse clinical translation to help set preclinical bars for advancing molecules
- What to expect in terms of animal modeling in the era of immune-based therapies
- An understanding of the potential of using client-owned animals (eg, dogs with cancer) for translational studies; when does it make sense?
- Examples of how these concepts have been reduced to practice to enhance the translatability of preclinical work and influence clinical development of compounds that have made it to market
Autoimmune diseases/inflammation (Dr. Rana Samadfam, Charles River Laboratories)
- Overview of in in vivo models used to characterize anti-inflammatory and immune modulatory drugs
- Overview and measurement of commonly studied mediators of immune and inflammatory responses.
- Therapeutic models of common interest for immune based diseases, including rheumatoid arthritis, psoriasis, asthma, inflammatory bowel disease/Crohn’s disease.
- Discussion of best practices for use of these models for evaluation of efficacy and mechanistic investiations
- Use of cell-based and molecular assays to characterize therapeutic responses.
- New and emerging technologies useful in characterization of immune and inflammatory responses.
- Strategic approaches for progression from early drug discovery screening to preclinical development, including use of biomarkers
Preclinical animal models (Dr. Leon Hall, Mousera)
- Limitations of current practices in preclinical research
- Developing more predictive models to address existing limitations
- Humanized mice in immuno-oncology applications
- Using humanized mice for other applications (safety/toxicity)
Metabolic Diseases (Diabetes/hyperlipidemia) (Dr. Peter Havel, UC-Davis)
- Review of established rodent models which have been used to study metabolic disease (obesity, insulin resistance and diabetes)
- Discuss limitations of monogenic rodent models which poorly represent the pathophysiology of these diseases
- Features of diet-induced obese rodent models of insulin resistance, which typically do not develop overt hyperglycemia sufficient to be classified as type-2 diabetes.
- Discussion of important differences between rodents in and primates with respect to metabolic physiology
- Characterization and advantages of the f UCD-T2DM rat model for studying type-2 diabetes, which combines polygenic obesity and insulin resistance with islet/ß-cell dysfunction leading to marked hyperglycemia and many of the typical features of type-2 diabetes in humans.
- Potential use of nonhuman primate models in drug development, such as the diet-induced rhesus monkey model of metabolic syndrome which exhibits insulin resistance, dyslipidemia and hepatic steatosis.
About the Speakers
Dr. Dirk Mendel is currently VP, Translational Biology, at E-Scape Bio, a local startup company focused on neurodegenerative diseases. He has almost 25 years of industry experience in preclinical research and early clinical development in several therapeutic areas. Dr. Mendel has held positions at a variety of companies including Gilead, SUGEN, Chiron, KAI Pharmaceuticals and MedImmune, the biologics arm of AstraZeneca, where he was VP and Global Head of Translational Medicine for all therapeutic areas. Dr. Mendel has extensive experience developing and successfully applying PK/PD and other translational approaches to facilitate preclinical and clinical development of targeted therapeutics in a variety of therapeutic areas. He has played a major role in progressing and/or leading multiple projects through research and early clinical development phases, including six compounds that have made it to market and a seventh that is currently under review in the US and Europe. His 2003 paper describing the PK/PD approach to understanding the activity of Sutent®, a multitargeted RTK inhibitor approved for the treatment of a variety of cancers, was recently highlighted by the senior editors of Clinical Cancer Research as one of the most important seminal articles they have published over the course of their 20-year history. Dr. Mendel received his Ph.D. in Physiology from Dartmouth Medical School and a B.S. in Engineering with a focus in Control Systems and Control Theory from Stanford University.
Dr. Stephen Gould is Director of Translational Oncology at Genentech, Inc. where he has overseen the preclinical pharmacology of their oncology pipeline for the past 9 years including four marketed drugs, Erivedge, Kadcyla, Pertuzumab and Venclexta. He obtained his B.S. degree at the University of Massachusetts, North Dartmouth in 1986 and his Ph.D. in biomedical science from the University of Connecticut, Farmington in 1993 where he discovered and characterized the role of a novel transmembrane heparin sulfate proteoglycan, Synecan-3, during limb bud development. Following his graduate work he joined the Laboratory of Robert Grainger at the University of Virginia as a postdoctoral fellow studying anteroposterior patterning of the central nervous system and how signals are passed through the plane of the tissue to establish polarity. He then moved to the West Coast for a additional postdoctoral training in the Orthopedic Surgery Department at UCSF in 1996 where he investigated the cellular contribution of autologous graft to bone fusion and characterized the proliferative response of osteoblasts to mechanical force in vivo using a novel small animal model of distraction osteogenesis. With a strong background in developmental biology and a renewed appreciation for translational science he then began his career in Biotechnology at Creative Biotechnologies, Inc. in Hopkinson, MA in 1998 where he researched clinical applications of Bone Morphogenic Proteins including a previously unappreciated role in reducing renal fibrosis following acute or chronic injury. Dr. Gould rose through the scientific ranks at Creative Biomolecules which became Curis in 2000 through a corporate merger, and led a collaboration with Genentech to develop small molecule Hedgehog pathway inhibitors for the treatment of cancer. At the termination of the research collaboration with Genentech, Dr. Gould followed the molecule that would eventually become Erivedge to Genentech where he played a pivotal role in its preclinical and clinical development. In 2012, Dr. Gould along with two of his Genentech colleagues shared a "Drug Discovery of the Year Award” presented by the British Pharmacological Society for their work on the pharmacology of vismodegib (Erivedge).
Dr. Rana Samadfam is a Scientific Director of In Vivo Pharmacology department at Charles River. She is also a principal scientist in Musculoskeletal Research department. She is a diplomat of ABT. She received her MSc and PhD degrees from the University of Sherbrook in Quebec Canada with focus on arthritis and pharmacology. Following her PhD, she accepted a post-doctoral scholar position at the University of McGill, Montreal Canada with focus on bone and endocrinology. She joined Charles River 10 years ago, accepting a position in musculoskeletal group. She is winner of several awards including the ASBMR Young Investigator Award and author of numerous peer- reviewed publications (over 80). Her area of expertise include inflammation, early in vivo pharmacology, toxicology, bone, diabetes and the endocrine system.
Dr. Peter Havel received his DVM and a PhD in Endocrinology from the University of California at Davis. He is a Professor with joint appointments in the Departments of Molecular Biosciences and Nutrition. Dr. Havel and his team investigate the regulation energy and carbohydrate/lipid metabolism, and the involvement of endocrine systems in the pathophysiology of obesity, diabetes, and cardiovascular disease. His laboratory is studying the mechanisms regulating the secretion of pancreatic, gastrointestinal, and adipocyte hormones. The role of endocrine, metabolic, and dietary factors in regulating energy balance, insulin action, and lipid/carbohydrate metabolism is examined in animal models and in clinical studies in humans. A major focus of the research is the interaction of diet composition (such as dietary fats and sugars) in the development and progression of obesity, diabetes, and dyslipidemia/atherosclerosis. His research team conducts studies on the prevention and treatment of diabetes in a rat model of type-2 diabetes (UCD-T2DM Rats) that is more similar to the pathophysiology of type-2 diabetes in humans and in a diet-induced rhesus monkey model of metabolic syndrome that exhibits insulin resistance, dyslipidemia, and fatty liver disease. Dr. Havel's laboratory also studies the endocrine effects of bariatric/gastrointestinal surgery and how hormones are involved in the improvements of carbohydrate and lipid metabolism observed post-surgery. He has published more 190 original peer-reviewed articles and 35 reviews and textbook chapters on his work in these areas.
Dr. Leon Hall is Global Head of Preclinical Operations at Mousera, a technology driven preclinical contract research organization in the Bay Area. He received his bachelors degree from the University of Guelph in Ontario Canada, before returning to his home country of England where he obtained his master’s degree and Ph.D. in Molecular Pathology and Toxicology from the University of Leicester. Leon moved to the US in 1999 accepting a post-doctoral scholar position at the University of California at Davis, specializing in characterizing cholinergic receptors in rodent models of dermatological disorders, and soon after he began providing core support to investigators in anesthesiology and neurodegenerative disorders research, transferring to a faculty appointment in 2003 and starting UD Davis’ spinal cord injury program. In 2005 Leon accepted a position as Study Director at The Jackson Laboratory where he helped build that program’s focus from metabolic disorders to include immunology, oncology, regenerative medicine, and neuromuscular and neurodegenerative disorders. While at JAX Leon headed up the program to commercially develop their humanized mouse immune system portfolio, and as Director of Scientific Operations he provided scientific and operations oversight to JAX's preclinical contract research service. Leon left JAX in 2013 to lead the development of Taconic Biosciences humanized mouse and patient derived xenograft programs, and as Sr. Director of Global R&D he was also responsible for leading their GEMs and microbiome research programs. Leon joined Mousera's senior leadership team in 2015.
Please go to below website for registration:
http://www.pbss.org/aspx/eventInfo.aspx?eID=505
SF Bay Area: Foster City Crowne Plaza

长三角绝佳区位
BioVillage, located on the southern bank of Hangzhou Bay Bridge, is in the vicinity of
four major cities of Shanghai, Hangzhou, Suzhou and Ningbo. It will become a shiny
star in the Yangtze River Delta Region.
The Yangtze River Delta Region, centered by the city of Shanghai, has embraced
many global pharmaceuticals and biotech companies, creating a superior culture and
atmosphere for drug research & development, commercialization, as well as
internationalized communication. It is emerging as a dense biopharmaceutical
cluster in China. Hangzhou Bay New Zone is right within the reach of this
leadingedge
settings and environment.
硬件设施完善
BioVillage, a garden style biotech park, with state-of- the-art design and facilities,
covers over 40 acres of land, providing 2.7 million square feet of lab space with
various functions, including office buildings, incubator space, GMP pilot labs, test
and analytical facilities, and SPF experimental animal vivarium.
政策扶持到位
The government of Ningbo Hangzhou Bay New Zone offers generous technical and
manpower support and tax benefits, stronger and deeper than the other domestic
development areas, in the effort to promote talent attraction and bringing in
innovative projects.
1. R&D incubator: Rental subsidization for up to 1500m2 (~160,000 SqFt) in the
first three years.
2. Innovation incentives: Tax exemptions, subsidizations and awards for High-
Tech Enterprises recognized by the Ministry of Science and Technology and
Ningbo government.
3. Talent policies: Relocation reimbursement ranging from RMB 150,000 to 3
million offered by the government; Housing allowance ranging from RMB
200,000 – 800,000 to qualified talents.
4. The “3315” project: Elected projects will be awarded RMB 5-20 million by the
government. The Hangzhou Bay New Zone will provide matching support at
various levels.
综合药研外包服务
In the seamless collaboration with Pharmaron, a world-class leading CRO, the
BioVillage is able to provide an integrative drug R&D platform from drug discovery,
development, to commercialization. This platform will provide biotechnology and
biopharmaceutical companies services in Synthetic, Medicinal and Analytical
Chemistries, Biology, Pharmacology, DMPK, Toxicology and Processing and
Manufacturing CMC.
Companies in the BioVillage can take full advantage of Pharmaron services with the
next-door advantage, and special discounted prices offered exclusively to the
BioVillage members.
一站式服务
The government is committed to provide a one-stop commodity to the incoming
companies and corporations. Various service executives are available to help you
establishing and developing your new entity. These services includes, but not limited
to, company registration, bank account opening, lab renovation, employee
recruitment and training, government relationship, government funding
opportunity/application, venture fund raising and patent application, etc.
多种基金配套
BioVillage will establish a venture capital of “BioPharma Development Fund”, with
stocking holdings not exceeding 40%. The fund also has close collaboration with
several national pharmaceutical and health funds, to provide additional funding
opportunities.
产业链配套便捷
Local resource for packaging materials, molds, parts and equipment – Zhejiang
provinces has several world-class markets for materials and small products.
Local resource for intermediates and APIs -- Zhejiang province is one of the largest
providers of API in China.
低成本优势
Much affordable housing and living cost (average housing price at RMB 5500/m2), to
ease your undertaking and bring your employees higher quality of life.
土地资源充足
Spacious industrial grade land will meet your demands for future expansion.
JOIN US NOW!
Please contact:
Dr. Sarah Chen sarahchen@biovillage-nb.com
Mr. Vincent Xu vicentxu@biovillage-nb.com
Ms Ella Shen ella@biovillage-nb.com
Ningbo, China
2016-5-21 浙江(硅谷)海外人才招聘和项目合作恰谈会
其中有十六家生物医药公司,名单见下。免费报名见下。
2016浙江(美国)海外高层次人才洽谈会将于5月21日和22日分别在美国硅谷和纽约举办。此次活动,将有近150家人才创业平台、知名企业、高校科研院所等单位,携带1500多个人才岗位需求和80多个项目合作需求来美现场交流、对接洽谈,并宣传、推介浙江的经济社会发展情况和留学人员创新创业环境。
其中有十几家生物医药公司
序号 参团单位 单位性质
27 海正药业(杭州)有限公司 生物医药
33 宁波沪信药物技术有限公司 生物医药
75 诸暨脉达生物科技有限公司 生物医药
78 浙江京新药业股份有限公司 生物医药
80 浙江加州国际纳米技术研究院绍兴分院 生物医药
81 浙江威宁益美生物科技有限公司 生物医药
109 浙江艾杰斯生物科技有限公司 生物医药
119 衢州邦华生物医药有限公司 生物医药
125 浙江海正药业股份有限公司 生物医药
126 浙江永宁药业股份有限公司 生物医药
128 浙江华海药业股份有限公司 生物医药
129 浙江康多利药业有限公司 生物医药
131 浙江仙居君业药业有限公司 生物医药
132 浙江仙琚制药股份有限公司 生物医药
134 浙江九洲药业股份有限公司 生物医药
149 浙江红珊瑚海洋科技有限公司 生物医药
一、时间地点
时间:5月21日上午10:00-13:00
地点:圣克拉拉会议中心(Santa Clara Convention Center)
地址:5001 Great America Pkwy., Santa Clara, CA 95054
早餐:活动将提供早餐;早餐时间9:00--10:00
二、活动报名
硅谷洽谈会的详情请浏览网站:http://tinyurl.com/zjsv2016
报名网站:http://tinyurl.com/zjsv2016form
报名选项时,请注明是通过CABS知道本次活动的.
请有意向参加对接活动的海外高层次人才尽早报名。一是有利于提前开始与有关企业对接, 提高对接效率; 二是有助于组织方预备早餐份数及其它准备工作; 谢谢您的大力支持!
三、举办单位
主办单位:浙江省人才工作办公室
浙江省人力资源和社会保障厅
浙江省人民政府外事侨务办公室
协办单位:北加州浙江大学校友会(硅谷)
中国旅美科技协会(纽约)
合作单位:美国浙江商会
海纳百川创业平台
热诚欢迎海外的高层次人才踊跃参会,共谋发展!恭请转发, 谢谢支持!同时也热诚欢迎有创业项目的高层次人才报名参加2016杭州海外高层次人才创新创业大赛:http://tinyurl.com/2016hzc
Santa Clara Convention Center, 5001 Great America Pkwy., Santa Clara, CA 95054
This Activity is cancelled due to weather condition, will reschedule to a later time
CABS Hiking Notice
Summer is coming, rain season is officially over, pollen is decreasing, and UV is going to be stronger soon later. Specifically, this Saturday is not going to be hot or cold. We need to have a CABS Hike!
That’s why this short notice comes to you. So, CABS Social Life Committee invites you to join us to have a traditional:
CABS Hiking, on May 21st 2016 Saturday. We will get together at 9 amand start to hike at around 9:15 am use the Ohlone College Park Entrance/Parking Lot G. You may park at Ohlone College parking lot, use the address here can bring you to the college: 43600 Mission Blvd., Fremont, CA 94539-5847.
If you are the first time to go there, please find the link about the park, trail and how you can prepare for the hiking: http://www.ebparks.org/parks/mission#parking_information. Please also check the Ohlone College map for the parking information. Since the parking space is limited (need pay fee too), carpooling is recommended.
It’s relatively challenging if you want to hike to the peak (8 to 10 miles). So, please be prepared and bring enough drinks and some light food. Your family members and friends are welcome, and please be aware that your safety is your responsibility.
You may scan the bar-code below and join the CABS hiking WeChat group to get more updates.
See you there. Thanks.
CABS Social Life
Scan the QR code to connect to this hiking event through Wechat!

Ohlone College parking lot, 43600 Mission Blvd., Fremont, CA 94539-5847.
2015年涉及中国实体企业的并购活动价值超 过160亿美元,
indicative of a growing buyer’s market and capital flow environment in China. There is no better time to forge cross-border partnerships than at ChinaBio®Partnering Forum 2016.
- How do I penetrate the fastest-growing healthcare market in the world and access China's prime dealmaking climate?
- What are the challenges of cross-border business?
- How do I position my asset with a multinational pharma (MNC)?
|
ChinaBio® Partnering Forum 2016 is co-produced by ChinaBio® Group and EBD Group, Inc. |
Registration Requirement:
CABS members enjoy a 20% discount on regular rate using ‘CABS20’ as Promo Code.
Organizers: ChinaBio Group; EBD Group
Kempinski Hotel Suzhou, Suzhou, China
As the BioPharma industry grows in China & the Asian region, we invite you to be a part of this tremendous investment & development opportunities!
The Biopharma Development & Production Week is the leading industry platform for pharma, biotech, CMOs, CROs, research institutes, investors, technology and industry stakeholders to meet, network and discuss current industry trends, establish business partnerships and be updated on investment opportunities in China and surrounding Asia.
More details about the event
Registration Requirement:
Grand Hyatt Shanghai, China

EPPIC GLOBAL Presents
A Partnering Event with Genentech
Global Perspectives for Payer Evidence and Impact on Pricing and Access
By Brian O’Shea and Jeff Hille
The global payer environment in the biopharmaceutical space is getting increasingly complex at a time when biopharmaceutical innovation is leading to unprecedented advances in treatment of difficult diseases. Regulators are increasingly more open to approving breakthrough medications, at a time when payers are struggling to manage increasingly tight budgets and growing demand for healthcare services. Brian O’Shea and Jeff Hille, from The Global Pricing and Market Access Group at Roche will discuss these challenges at a global level, and will specifically describe how these challenges are addressed in key individual countries.
About Brian
Brian is Director in the Global Pricing and Market Access group at Genentech/Roche. He leads a team of 6 international payer strategy leaders responsible for pricing and global market access for Alectinib, Zelboraf, Erivedge, Cotellic, Tecentriq and market preparation for several early molecules. In his 8+ years at Genentech/Roche, he has had a variety of leadership experiences and roles, including starting the Reimbursement Strategy Team in Managed Care and Customer Opetarions, International Payer Strategy Leader on multiple molecules including Zelboraf and Erivedge, and Channel and Access Solutions Marketing team lead. He has over 25 years of industry experience and has previously worked at GSK, Astrazeneca, and Merck.
About Jeff
Jeff is Senior International Payer Strategy leader: Oncology Pipeline in the Global Pricing and Market Access group at Genentech/Roche. He joined the company over 8 years ago and held leadership positions in the Managed Care and Customer Operations, and Health Economics and Outcomes Research groups, and as the International Payer Strategy Leader on Perjeta. Jeff has over 14 years in of industry experience and has previously worked at Eli Lilly.
About Genentech and EPPIC
Genentech, a pioneer in Biotechnology, is well known for its investment in innovation and the principles of partnering, mentoring and networking within its organization as well as the wider life sciences community.
EPPIC GLOBAL has enjoyed partner events and sponsorship support from Genentech for over a decade. In 2016, EPPIC is proud to announce that Genentech was one of the Platinum Sponsors to the annual flagship conference – EPPICon 2016. Several leaders from Genentech were at the event and many symbiotic interactions have already begun.
Come join EPPIC today!
EPPIC strives to bring its members partnering and enriching events that synergize the values and principles of the organization.
Register FREE for this event at: https://www.123signup.com/register?id=ngrjv
When: Friday, May 13, 2016 12:00 -1:00 PM
Where: Genentech Campus - Building 42-1C, 455 E. Grand Avenue, South San Francisco, CA 94080
Lunch will be provided from 11:30 onwards
Free Registration ends on Friday, May 09, 2016
Genentech Campus - Building 42-1C, 455 E. Grand Avenue, South San Francisco, CA 94080
Inaugural BioPacific Partnering Forum
The 2016 BioPacific Conference brings together leaders in the life sciences community from the Pacific Rim and the United States. In order to further facilitate the interactions including project introduction and talent recruitment between our attendees and our sponsors, we are introducing BioPacific Partnering Forum in conjunction with our conference this year.
BioPacific Partnering Forum on May 8 Sunday (the next day after BioPacific Conference) for project collaboration and funding, and talent recruitment.
This is a complimentary event for BioPacific Conference attendees, sponsors and exhibitors.
Please use the link below to register. Registration is free.
https://docs.google.com/forms/d/1BQ_Q3GY53oyuOLYucsjPE_Df3IYxOU8ibRs-hPQXYKM/viewform
Agenda:
9-9:20am Networking
9:20-10:20am Featured presentations from three major sponsors regarding project collaboration and talent recruitment
- · Ningbo Hangzhou Bay New Zone, 宁波杭州湾生命科技园(BioVillage)
- · Zibo National High-tech Industrial Development Zone, 淄博高新区生物医药产业创新园
- · Chengdu Kanghong Pharmaceuticals Group, 成都康弘药业集团股份有限公司
10:20-10:50am Brief presentations from other sponsors including Sekisui XenoTech, Paragon Genomics, JenKem Technology, Kelun Pharmaceutical 科伦药业, and more later
10:50am – 5pm Small group meetings or 1/1 discussions (may finish earlier than 5pm)
Information of three major sponsors:
- 1. 宁波杭州湾生命科技园(BioVillage)
- a) “宁波杭州湾生命科技园(BioVillage)” 位于国家级经济技术开发区-宁波市杭州湾新区,在“东方巨龙”——杭州湾跨海大桥南岸,是上海、杭州、苏州等大城市的几何中心,长三角的明日之星。项目总投资56亿元,规划用地面积16.7公顷,将建成25万平方米集创业孵化楼、企业独栋、GMP中试实验室、分析测试平台、实验动物平台等组成的国际化、花园式生命科技园区。入园企业足不出户就可以在其个性化设计的服务平台上,获得从研发到产品上市全过程的所有配套设施支持和零距离、国际一流的专业化的技术服务。让入园者以更低的创业成本、更短的创业周期达成创业成功。园区同时预留稀缺的2000亩生物医药工业成熟用地,满足企业扩张用地的需求。
b) 项目,企业和人才需求
1) 项目:生物科技,生命健康等领域,包含但不限于生物医药,医疗器械,检验检测,生物环保,农业等领域的项目;
2) 成熟企业:拟在中国发展,落地,建立厂房,研究中心,工作机构,办事处等
3) 院所:拟在园区建立联合实验室,研究开发平台,产业转化平台等
4) 人才: 带项目或团队回国的生物技术类创业者人才。
2. 淄博高新区科技创新创业资源平台与创新技术需求
- a) 淄博高新区生物医药产业创新园是国家生物医药产业基地, 设生物技术药物、天然药物、化学合成药物三个功能研发楼和中试车间,为生物医药项目开发与孵化提供完善的支持体系。公共技术服务平台一期建设占地4500㎡,拥有大中型仪器设备487台(套),仪器总投资5000余万元。平台下设药物分析测试中心、技术资料信息查询中心、生物技术药物实验室、化学合成药物实验室、天然药物实验室、药物制剂实验室和制药过程分析实验室(建设中)七个功能单元。山东大学淄博生物医药研究院的主要职责是平台运营、技术服务、成果转化与孵化、人才培养、交流合作。对外提供:技术服务资源共享、项目(技术)委托开发、成果转移-转化-孵化与市场推广等技术支持。
- b) 技术和项目需求: 生物医药、健康医疗、大数据开发与应用、新材料、先进制造、节能环保等领域为主;条件是以产业化为目标,科技含量高,技术成熟,市场前景较好,知识产权明晰无纠纷。
3. 成都康弘药业集团股份有限公司
- a) 成都康弘药业集团股份有限公司是一家致力于中成药、化学药及生物制品的研发、生产、销售及售后服务的上市医药集团,总部位于中国四川省成都市,现有员工4000余人,拥有中成药、化学药和生物制品生产基地,销售网络遍布全国。在中枢神经系统、消化系统、眼科及其他等重点临床领域均有上市和处于研发阶段的创新产品,已申请和授权专利达到160余项(国外专利40项)。
- b) 项目需求:在中枢神经系统、消化系统、眼科及其他等重点临床领域有知识产权的创新项目,尤其是满足临床需求的特色小分子化学制剂,创新生物大分子药物等,具体情况请面洽。
- c) 人才需求:化学药物和生物大分子药物研发,生产工艺,质量控制,申报等领域的高端专业人才,具体情况请面洽。
Information of other sponsors including Sekisui XenoTech, Paragon Genomics, JenKem Technology, Kelun Pharmaceutical, Qilu Pharmaceutical and more
Sekisui XenoTech
Xenotech.com, 1101 West Cambridge Circle Dr., Kansas City, KS 66103
- 1. Sekisui XenoTech is a CRO with locations in Kansas, USA and Tokai, Japan, specializing in-vitro drug metabolism studies, many of which are required by the FDA an EMA in their ddi guidance documents for drug approval (reaction phenotyping, CYP inhibition, CYP induction, drug transport, metabolite ID, PPB, etc), in vivo AAALAC accredited ADME studies, a full line of drug metabolism products (microsomes, hepatocytes, etc), JCRB cell lines (~1200 cell lines), radiolabeled compound synthesis and metabolite generation.
- 2. Sekisui XenoTech would like to collaborate with new pharmaceutical companies to provide ADME and DMPK services or products.
- 3. Sekisui-XenoTech has a need for scientist experienced with Metabolite Identification.
Paragon Genomics: South San Francisco
- 1. Headquartered in South San Francisco, Paragon Genomics develops and commercializes fast, easy-to-use, and highly uniform multiplex PCR technology and products for targeted sequencing in the Next-Generation Sequencing (NGS) and precision medicine markets. Our CleanPlex™ technology employs a proprietary background cleaning mechanism that effectively removes non-specific PCR products generated during multiplex PCR reactions, dramatically improving the accuracy and speed of targeted sequencing. Our technology is applicable to many fast growing NGS segments such as cancer diagnostics, non-invasive prenatal testing (NIPT), inherited disease testing, liquid biopsy and personalized medicine.
- 2. Company Needs for Project Collaboration: Paragon Genomics is looking for Next Generation Sequencing labs that are interested in joining our early access customer program to apply CleanPlex™ to various segments of the fast growing molecular diagnostics and precision medicine markets. We also welcome discussions about potential opportunities to distribute our targeted sequencing reagent kits in selected geographical areas such as China, South America and Europe.
- 3. Recruiting Needs: bioinformatics specialists with experience in NGS bioinformatics pipeline and multiplex PCR assay design.
JenKem Technology USA Inc.
4105 W. Spring Creek Pkwy, #606B, Plano, TX 75024, U. S. A.
- 1. JenKem Technology specializes in the development and manufacturing of high quality polyethylene glycols (PEGs), monodisperse discrete PEGs, PEG co-polymers, custom PEG synthesis, and PEGylation services.
- 2. JenKem Technology offers high quality PEGs and PEGylation services to the pharmaceutical, biotechnology, medical device, diagnostics, and emerging chemical specialty markets, from laboratory through commercial GMP scale.
- 3. JenKem Technology is always looking for talent in research, manufacturing and analysis of polyethylene glycols at our Beijing and Tianjin sites, and for local talent in the Dallas Fort Worth area for our sales and marketing office.
Kelun Pharmaceutical
- 1. 科伦集团创立于1996年,现已发展成为拥有海内外90余家下属企业的现代化药业集团,是中国医药行业的领军型企业。集团旗下的科伦医贸和科伦药业(股票代码:002422)双双进入全国医药流通业和制造业前10强。2014年,集团营业收入超过350亿元,利税超过20亿元人民币。集团现有药物研发人员700余人,构建了以中央研究院(成都)为核心,以南方(苏州)、北方(天津)研究分院、海外(美国新泽西、圣地亚哥)研究分院为分支机构的集约化研究体系;致力于肿瘤、糖尿病、肝炎、心血管疾病等重大疾病领域的品牌仿制药、创新小分子、新型给药系统和生物技术药物的研发。
- 2. Talent recruitment: over 30 job positions in China and in the US in the above areas including innovative small molecule, biosimilar, etc.
More sponsors later
Hanqi Investment Inc. at 1633 Bayshore Hwy, Unite 280, Burlingame, CA 94010
Chinese-American Biopharmaceutical Society (CABS) K. Fong Special Award
Dr. Gerald Chan to be Honored with CABS K. Fong Special Award - Visionary in Life Sciences
CABS Public Announcement: March 31st, 2016.
Chinese-American Biopharmaceutical Society (CABS) K. Fong Award Committee is very pleased to announce that Dr. Gerald Chan is the winner of its CABS K. Fong Special Award - Visionary in Life Sciences for his extraordinary vision and leadership in cultivating a generation of successful entrepreneurs and life sciences companies. Dr. Gerald Chan will be presented with this special award and we are honored that Dr. Chan will also be speaking at the 2016 BioPacific Conference (18th Annual Meeting of CABS) on 7th May 2016 at the San Mateo Marriott San Francisco Airport, 1770 South Amplett Blvd., San Mateo, CA 94402.
Dr. Gerald Chan is the co-founder of Morningside, a private investment group with venture capital, private equity and property investments. In the life science sector, Morningside focuses on start-up biotechnology companies founded on novel scientific discoveries. Notable investments in oncology that Gerald led include novel approaches to cancer therapeutics such as oncolytic viruses (BioVex, DNAtrix), cancer vaccines (Aduro), modified cytotoxic agents (Nucana) and novel targets for therapeutic intervention (CellCentric, K-Gen, Vigeo). Investments in the infectious disease area include novel antibiotics (MicuRx, Artugen), prophylactic vaccines (Matrivax) and antivirals (Atea). Other investments cover the therapeutic areas of metabolic diseases (CVI), autoimmune diseases (Kezar), CNS disorders (Orthogonal, Synchroneuron, Cognoa), and rare orphan diseases (Stealth, Apellis), as well as molecular diagnostics (ACD), drug delivery (Medtrum, Incarda) and medical devices (ViOptix, Kona, Excera). Gerald is a member of the advisory boards of the International Society for Stem Cell Research, the New York Academy of Science, Cold Spring Harbor Conferences Asia, Johns Hopkins Nanjing Center, and Columbia University Center for Radiological Research. At Harvard University, he is a member of the Global Advisory Council, the Dean's Board of Advisors of the Harvard T.H. Chan School of Public Health and the Harvard China Fund. He chairs the Overseers Committee of Morningside College of Chinese University of Hong Kong.
Gerald received his BS and MS degrees in engineering from UCLA, his Master's degree in medical radiological physics and Doctor of Science degree in radiation biology from Harvard University. He received his post-doctoral training at the Dana-Farber Cancer Institute as a fellow of the Leukemia Society of America. The Chinese University of Hong Kong and the University of Glasgow have conferred on him honorary degrees. He was elected to an honorary fellowship at Wolfson College of Oxford University. In 2012, he was the commencement speaker of the Harvard T.H. Chan School of Public Health.
“CABS is very proud to honor Dr. Gerald Chan for his life-time contribution in the field of life sciences, his significant achievement towards nurturing a new generation of innovative life sciences companies and his generous support of life sciences education, including a transformative gift of $350M to the Harvard School of Public Health, the largest donation in the 380-year history of the university. ” said Dr. Jiangwen Majeti, President of CABS. “ We are very grateful to Dr. Ken Fong for his generous long-term support of this award as well as other activities at CABS throughout the year.”
About CABS K. Fong Award in Life Sciences:
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life. Candidates must be nominated by an active member of CABS followed by review and approval of the Selection Committee. Selection criteria will be based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or the research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the general biomedical and the CABS community and to promoting international collaboration in life sciences.
About CABS:
Chinese American Biopharmaceutical Society (CABS) is a non-profit organization for professionals in the biopharmaceutical industry, headquartered in San Francisco, California with over 3,000 members and participants. While many of our members are located in the San Francisco Bay Area, the largest biotechnology hub of the world and the birthplace of biotechnology, we also have members in other parts of the U.S. and in the Pacific Rim countries including China. Our missions include the following:
- To serve biopharmaceutical professionals of Chinese heritage or with professional interests in China, and promote professional interactions.
- To foster business opportunities and exchanges in the life science industry between the U.S. and China.
- To promote public awareness of progress and development in the pharmaceutical and biotechnological industry.
- To collaborate with other organizations in areas of mutual interest.
About Dr. Kenneth Fong:
Dr. Kenneth Fong is currently the founder and chairman of Kenson Ventures, LLC, a company that specializes in crafting development strategy for early and mid-stage stage biotech companies. Under his leadership, companies that were acquired or went public between 2007 and 2012 are: SA Biosciences (to Qiagen), DHI (to Quidel), Fermenta (to ThermoFisher), Panomics (to Affymetrix), Epitomics (to Abcam), Bioform (IPO) and Optimer (IPO). Currently, he sits on the boards of 5 biotech companies, including two biopharmaceutical, one diagnostic and two research tool company. Prior to Kenson, Ken founded and served as CEO of Clontech Laboratories (1984-1999), which was acquired by Becton Dickinson in 1999. Clontech, a leader in the life science market, was also the largest of its kind founded by an Asian American (400 people, including 65 PhD scientists) during the early years of the biotech industry. Ken served as president of Society of Chinese Bioscientists in North America (~2,000 members, 2005-09) and President of the Bay Area AAMA (1987). He was a member of the Board of Trustees of California State University (2006-2013), Board of Associates at the Whitehead Biomedical Institute at MIT and a member of the Committee of 100 (US). Ken has many other philanthropic interests. He was one of the lead supporters for the San Jose Tech Museum, the Chinese Historical Society in San Francisco, the Bioengineering Auditorium at UC San Diego and the Indiana University graduate Seminar Programs. He has provided Translational Science Awards through an endowed fund to the San Francisco State University. In addition, he has provided a number of scholarships to Peking University and the CSU students (Kenneth Fong–Hearst endowed Scholarships). In 2006, he was also involved in establishing the Fong Optometry and Medical Library at UC Berkeley and more recently an endowed professorship to Stanford University. Currently Ken spends a good amount of time cultivating and advising a number of entrepreneurs (40-55 years old group) to grow their biotech companies.
Past recipients of the K. Fong Awards include:
2015: Dr. Irving Weissman, Stanford University for his pioneering research in stem cell research.
2014: Dr. Ge Li of Wuxi Apptec for creating and shaping the CRO business model in China and Dr. Hing L. Sham, formerly of Eli Lily, for his leading role in the discovery of life-saving HIV protease inhibitors, ritonavir and Iopinavir.
2013: Dr. Peter Hirth of Plexxicon & Sugen for his pivotal role in advancing 4 successful drugs to the market and Dr. Jean Cui, formerly of Pfizer for her role as the lead designer and investigator of crizotinib, a successful kinase inhibiting drug used in personalized medicine.
San Mateo Marriott, 1770 South Amphlett Blvd, San Mateo, CA 94402

BioPacific Conference 2016
18th Annual Conference of the Chinese American BioPharmaceutical Society
&
Inaugural BioPacific Partnering
Click below to see conference program book
BioPacific 2016 Program Book Final.pdf
Our meeting will showcase:
- Winner of 2016 CABS K. Fong Award in Life Sciences: Gerald Chan, Sc.D., co-founder, Morningside
- Success stories of entrepreneurs and emerging companies including Adheron Therapeutics and PaxVax
- Most promising areas in life sciences including cancer immunotherapy and CRISPR/dCas9 technology
- BioPharmaceutical Development - China & Global Considerations
- US-China Cross-Border Healthcare Investment & Entrepreneurship
- Two-day BioPacific Forum to facilitate interactions including project introduction and talent recruitment between attendees and sponsors
Confirmed Speakers and Panelists include:
• Gerald Chan, Sc.D., Co-Founder, Morningside
• Hari Kumar, Ph.D., Ex-CEO & BOD, Adheron Therapeutics
• Piers Whitehead, A.B., EVP & CBO, PaxVax
• Mark Selby, Ph.D., Senior Director of Immuno-Oncology, Bristol-Myers Squibb
• Stanley Qi, Ph.D., Assistant Professor, Stanford University School of Medicine
• Darren Ji, Ph.D., M.D., M.B.A., Vice President and Global Head, Roche Partnering Asia and Emerging Markets
• Tom Irving, J.D., Partner & Wen Li, Ph.D., Associate, Finnegan, Henderson, Farabow, Garrett & Dunner, LLP
• Robert Lou, Manager Director, Ningbo Kangtaibo Technology Development Co. Ltd
• Hui Zhang, Deputy Director, Ningbo Hangzhou Bay New Zone Investment and Partnership Branch
• Jinqun Yin, M.B.A., Vice President, Chengdu Kanghong Pharmaceutical Group Co., Ltd
• Charles C. Comey, J.D., Partner, Morrison & Foerster
• Shawn Qian, Ph.D., CEO, Neupharma
• Xiaodong Yang, M.D., Ph.D., President and CEO, Apexigen
• Xian-Ping Lu, Ph.D., Co-Founder, CEO and CSO, Chipscreen Biosciences
• Dan Zhang, M.D., Ph.D., CEO, Fountain Medical Development
• Connie Sun, Ph.D, Senior Vice President, Business and Corporate Development, Pharmaron
• Cheni Kwok, Ph.D, CLP Senior Consultant, Medicilon, Managing Partner, Linear Dreams
• Alexis Ji, Ph.D., M.B.A., Principal, WuXi Healthcare Ventures
• James Zhao, M.D., M.S., M.B.A., Founding Partner, LYFE Capital
• Yuwen Liu, M.B.A., MS, Founding Partner, BoHe Angel Fund
• Min Cui, Ph.D., Founding Partner and Managing Director, Decheng Capital
• Vincent Liu, Ph.D., M.B.A., Senior Consultant, Wanbang Biopharmaceutical Shanghai Fosun Pharmaceutical (Group) Co., Ltd
• Shawn Lee, Ph.D. Vice-Chairman, Xinbang Pharmaceutical, President & CEO, CPC Scientific Inc.
• Cheng Liu, Ph.D., Founder and CEO, Eureka Therapeutics
• Jennifer Hu, Ph.D., Venture Partner, Qiming Venture Partners
• Steven Adams DVM, Ph.D., Executive Director, Portfolio & Operations for the Oncology Research Worldwide R&D, Pfizer
See conference agenda at: http://biopacificconference.org/
More information on one-on-one meetings at:
http://www.biopacificconference.org/partnering/
Registration:
Refreshments, lunch and dinner are included for all registrants
| CABS Member | Early Bird Registration (before 04/15/16) | Regular Registration (04/16/16 - 05/04/16) | Late Registration (after 05/04/16)* |
| General | $65 | $100 | $100 |
| Student/Postdoc** | $50 | $80 | $80 |
| Non-Member | Early Bird Registration (before 04/15/16) | Regular Registration (04/16/16 - 05/04/16) | Late Registration (after 05/04/16)* |
| General | $125 | $160 | $160 |
| Student/Postdoc** | $110 | $140 | $140 |
*No guarantee on lunch and dinner
** A valid .edu email address is required for student/postdoc price
Join CABS today to save $ on registration fee: http://www.cabsweb.org/members/
Questions? Email: info@cabsweb.org
We are looking for volunteers to help during the conference. Volunteer will get free conference admission. Email: linyanbio@
Want to be a sponsor or vendor? Email: fundraising@cabsweb.org

San Mateo Marriott, 1770 South Amphlett Blvd, San Mateo, CA 94402
Naturejobs Career Expo - San Francisco
Date: April 27, 2016 @ 10:00am – 5:00pm
Location: Contemporary Jewish Museum (736 Mission St, San Francisco, CA 94103)
The Naturejobs Career Expo is the global career fair and conference for the science community from Naturejobs and Natureconferences. The Naturejobs Career Expo 2016, hosted for the first time in San Francisco, offers talented researchers an excellent opportunity to meet a diverse selection of national and international employers from academic institutions and scientific industries, such as pharmaceutical organizations, digital technology companies, science publishing and more.
• Attend all conferences and workshops for free
• Meet employers face-to-face
• Benefit from one-to-one resume checking
• Network with leading scientific institutions
• Learn how to enhance your employability
• Maximize your career prospects
Contemporary Jewish Museum (736 Mission St, San Francisco, CA 94103)
Free registration for everyone!
List of speakers
Dr. YK Zhang, Discovery of Benzoxaborole Antimalarial Agents, Research Leader of Anacor
Kerydin® (Tavaborole), approved by the FDA in July 2014 for the topical treatment of onycomycosis of the toenails, is one of the bioactive clinical compounds YK Zhang and his colleagues discovered for Anacor Pharmaceuticals, Inc., Palo Alto, California. YK has worked in many biotech companies in the Bay Area, and has conducted research for Anacor since 2003. His experience includes leading medicinal chemistry teams for HCV, malaria, and three animal health discovery programs, managing chemistry outsourcing with international CROs and integrating medicinal chemistry research activities for discovery programs. In the course of his research, he has contributed to 54 patents and applications, 66 peer-reviewed scientific research articles and presented at 46 conferences.
Dr. Henry Johnson, Targeting the Immunoproteasome, Principal Scientist of Kezar Biotheapeutics
Henry W. B. Johnson is a Principal Scientist at Kezar Life Sciences, a startup focused on developing innovative new therapies targeting protein homeostasis. Prior to joining Kezar he was a senior member of the medicinal chemistry team at Onyx Pharmaceuticals and Exelixis. Over the course of his career he has led or been a primary contributor on many successful project teams including the development of five small molecules which entered the clinic. He obtained his PhD in the laboratory of Jon Rainier at the University of Utah where his work was focused in the area of natural product synthesis and methodology. He earned his B.S. in biochemistry from Cal Poly, San Luis Obispo. Dr. Johnson is an author on more than 40 scientific publications and patents.
Dr. Leping Li, Expanding the HCV Infection Treatment Options through Chemistry, VP of Assembly Biosciences
Leping Li, PhD, has more than 20 years of experience in drug discovery and early development, leading project teams and advancing multiple programs from early lead identification to advanced clinical development in infectious diseases, cancer and metabolic disorders. Leping joined Assembly Biosciences as Vice President, Discovery this year. Prior to this position, he was with Presidio Pharmaceuticals, where he was Vice President of Chemistry and inventor of the HCV NS5A inhibitor ravidasvir. Previously, he was Scientific Director in Medicinal Chemistry at Amgen, where he received the 2004 Robert A. Swanson Distinguished Employee Award. Before Amgen (and Tulrik), Dr. Li served as a Research Investigator at Abbott Laboratories. Dr. Li obtained a BS degree from Shandong University in China. He earned a PhD in organic chemistry at Rice University and conducted postdoctoral research at Stanford University.
I wish to express my deep gratitude to speakers’ kind support and time to prepare the seminar. The presentation will provide great benefit to the CABS community. We will have a great event!
Hanqi office, 1633 Bayshore Hwy Ste 280, Burlingame, CA 94010
Application Open: CABS Career Advisory Network (CAN) Program 2016
Application Link: http://goo.gl/forms/1Ks19ytg2M
Deadline: 04/16/2016, 11:59 pm (PST)
Mentor List and Schedule: Click Here
The CAN program aims to cultivate CABS members to be the next generation of life science professionals and leaders through mentoring, skills building, and networking. It is a six-month program with 1:1 mentoring meetings and group sessions. We have lined up 30 mentors (click here for mentor bios) for 2016 with diverse expertise. Once matched, mentees will meet with their mentor monthly to discuss specific career-building topics. As part of the process, participants will create a personal strategic plan for identifying ways to achieve career goals. Except for the 1:1 mentoring, CAN program will also host Brunch/cafe gathering, mock interview, and panel discussion to facilitate the networking among the participants and peer mentoring platform. Please check here for the detailed 2016 Schedule and mentor bio.
CAN program has been successfully held for the past six years and received many positive feedbacks. It is an excellent opportunity to interact and network with a diverse group of people in academia and industry in a supportive and informal setting. 2016 CAN program will continue to provide our members an interactive relationship with mentors discussing personalized career issues and a platform for supporting, networking and career development.
How to Apply?
1. Become an active CABS member. (CABS membership application link)
2. Complete the CAN application before April 16th, 2016 midnight (Registration Link). We will verify your CABS membership during review of your application.
3. Applicant screening and matching (with optional phone/in-person interview if requested by mentor) between April 16th and April 30th, 2016
4. Acceptance will be notified by late April to early May
Contact Information:
Please contact bcd@cabsweb.org if you have any question about the application.
Registration Requirement: Active CABS membership required
Organizers: CABS Business and Career Development Committee
Online application
San Francisco Bay Area Startup Competition Preliminary in Life Science
If interested in participating in the competition, please go to CLICK HERE TO APPLY under "how to participate". The application deadline is March 15, 2016
If interested to be an audience, please go to REGISTER at the top or bottom.
For more information, please email BSK’s US contact at icc@cabsweb.org
Nanjing BioSciKin Co., Ltd (BSK), an open investment and incubation platform in China for life science innovation is sponsoring an international startup contest in life science. The co-sponsors include influential Chinese industrial investors, pharmaceutical companies and Chinese media with the support of a powerful alliance of strategic investors. The event runs from December 2015 to June 2016. It will take place in 8 cities in both China and the US, where the US preliminaries will be held in San Francisco and Boston. The final competition will be in Shanghai.
Highlights:
• Your BP will be exposed to many investment firms including BSK, Hony Capital, Fosun Pharma, Trust Bridge Partners, Ally Bridge Group, GT Capital, PointGuard Ventures, Bohe Ventures, Tenyall Investment, Firestone Investing, Spruce Capital Partners and BAR Capital Management.
• The investment can be in the US or China. It is not mandatory to set up China presence. The above firms have invested in many life science companies in the US and China.
• The investment can be, but not limited to, $500K to $10 million.
• All BPs submitted before deadline of March 15, 2016 will get feedback by email from judges and investment firms. If your BP receives good reviews, you will have meeting with investment firms.
• 1st place company in each preliminary will receive $30,000 in non-dilutive financing. 1st and 2nd place companies in each preliminary will receive a paid trip to Shanghai to present at the final in front of many investors.
Who can participate: The event welcomes both companies and entrepreneurial teams in life science area.
How to participate:
- Application Deadline is March 15, 2016: please send an executive summary or no more than 10 slides in either PowerPoint or as a PDF along with your contact information below.

- About 10 startups will be selected to showcase their projects at preliminaries. You will receive a formal invitation to the preliminaries. Two teams with the highest ratings from the judge panel will advance to the final.
For more information, please email BSK’s US contact at icc@cabsweb.org
Agenda:
|
6:00-6:30pm 6:30-6:40pm 6:40-7:10pm |
Registration Open remarks Lecture by keynote speaker: “Noninvasive liquid biopsy for cancer patient” |
All CABS Dr. Lin Wu |
|
7:10-8:40pm |
Project road show part I (10 mins presentation; 5 mins Q&A each) |
5 contestants |
|
8:40-8:50pm |
Tea break |
|
|
8:50-10:20pm |
Project road show part II (10 mins presentation; 5 mins Q&A each) |
5 contestants |
|
10:20-10:40pm |
Announcement of preliminary results and prize presentation |
The sponsors, judge panel |
Keynote Speaker:
Dr. Lin Wu
Dr Lin Wu is Vice President, Development for the Sequencing Business Unit of Roche Diagnostics. Dr. Wu has strong background in IVD assay development and registration. She led the development of the first microarray-based pharmacogenetic assay approved by the FDA, AmpliChip CYP450, and assumed various leadership roles during the development of the cobas oncology portfolio. Dr. Wu pioneered the scientific research work in detecting tumor DNA in blood, which eventually led the launch of cobas EGFR blood assay. This work was the first recipient of Roche Diagnostic DIAmond Award for R&D Innovation.Dr. Lin Wu received her Ph. D. in Molecular Biology from State University of New York at Buffalo. Prior joining Roche, she completed her post-doctoral fellowship in cancer biology at Princeton University.
Panel of Judges:
Pete Thomas
Pete Thomas is a Managing Director of PointGuard Ventures(PGV). Prior to co-founding PointGuard Ventures, Pete co-founded ATA Ventures in 2004, a firm that raised $440M in capital across three funds during the last ten years. ATA has invested in over 40 early stage companies and Pete was on the board and directly involved in 11 of those companies and is presently on the board of Revera, Lavante, and Adesto Technologies and is an advisor to Theranos.
Krish Panu
Kris panu is a Managing Director of PointGuard Ventures(PGV). For more than two decades Krish has built successful technology businesses which have created multi-billion dollar markets. Prior to entering the venture capital industry, Krish has held executive positions in several influential companies including @Road, Inc., Atmel, Catalyst Semiconductor, Datapoint Corporation and Xicor etc.
Roger Wyse
Dr. Roger Wyse is the Managing Partner of Spruce Capital Partners and BAR Capital Management. He chairs or serves on the boards of 11 private companies. He is Co-Chairman of the $162M Malaysian Life Sciences Capital Fund. Heis also an internationally recognized scientist and was a Dean at 2 major research universities. He was founder and Chairman of the Alliance for Animal Genome Research. He is on Board of Industrial Section of BIO.
Luke Li
Dr. Luke Li has 30 years of experience in biopharma and healthcare business. Currently, Dr. Li is a Managing Director at Ally Bridge Group (“ABG”), a global healthcare investment platform. Before joining ABG, Dr. Li has held Executive positions at Pfizer and Amgen. He was an Assistant Professor in Medicine at Washington University Medical School.
Jason Wen
Jason Wen is the Director of Technology Transfer at Boston College. He has a broad background combining experience in the life sciences, business, and patent law. Dr. Wen is a long time member of the Association of University Technology Managers (AUTM) and the Licensing Executives Society (LES). He is also a Registered Technology Transfer Professional (RTTP) and a United States Patent and Trademark Office Registered Patent Agent.
Michael Draper
Sanofi North America’s Head of External Science and Partnering on East Coast.
Other judges include delegates from BioSciKin
BioSciKin is actively seeking investment opportunities in precision medicine filed and has made more than 10 investments over past two years in US, Korea and China, with typically investment size ranging from 0.5 million to 10 million USD.
Organized by: Chinese American Biopharmaceutical Society (CABS) and Association of Chinese Students and Scholars at Stanford (ACSSS); supporting organization: California Life Sciences Institute (CLSI)
Sponsored by: Simcere Pharmaceutical Group; BioSciKin; Simcere State Key Laboratory of Translational Medicine and Innovative Drug R&D; HEALTHCARE EXECUTIVE Magazine; Firestone Investing
About Simcere Simcere is an emerging leader in developing, manufacturing and marketing of innovative medicines and branded generics in China. Simcere was the first Chinese chemical and biological drug company to list on the NYSE in 2007. In 2013, the company went private for strategic considerations. In 2015, Simcere’s translational medicine and innovative drug R&D platform has been recognized as a State Key Laboratory by the National Ministry of Science and Technology. The company has been actively pursuing cross-border partnerships to bringing global innovation to China. In Boston and New Jersey, Simcere has two offices in the US for accelerating international business development.
About BioSciKin BioSciKin (BSK) is an open platform in China for life science entrepreneurs and innovators. Focuses on three major healthcare areas: novel therapeutics, medical devices and mobile healthcare services, BSK is looking for partnership from all over the world for co-development of innovative approaches in precision medicine. In Nanjing, Shanghai and Tianjin, BSK has established 3 R&D-oriented incubation parks with advanced R&D facilities to provide entrepreneurs with customized service and support packages. For more information, please go to BSK’s official website: www.bioscikin.com. You can also email to Ms. Ray Zhang, Senior BD director, BioSciKin (zhangruikun@bioscikin.com)
About HEALTHCARE EXECUTIVE Magazine HEALTHCARE EXECUTIVE Magazine is an influential magazine in the Chinese healthcare industry.
About Firestone Firestone focuses on innovation and entrepreneurship services in healthcare industry.
Stanford University, Room130, Li Ka Shing Learning and Knowledge Center, 291 Campus Drive, Stanford, CA 94305 (map at the bottom, light dinner provided)
Click here for details
Grand Hyatt Shanghai, Jin Mao Tower, 88 Century Boulevard, Pudong, Shanghai, China 200121
RSVP Link: http://goo.gl/forms/DBlBZK0Tt8
Agenda:
1:30 - 2:00pm Registration and Networking
2:00 - 3:00pm CAN Program Introduction
Mentor Introduction
3:00 - 3:30pm Q&A Session
3:30 - 4:00pm Mixer with mentors
*Drinks and light refreshment will be provided
In keeping with its mission of engendering professional interaction and development within the biotech/pharma industry, the Chinese-American BioPharmaceutical Society (CABS) is offering a free mentoring program, Career Advisory Network (CAN), to help advance career goals of its active members.
CAN is a six-month program with 1:1 mentoring meetings and group network sessions. We have lined up an exceptional group of mentors for 2016 with diverse expertise and experiences encompassing pharmaceutical R&D, biotechnology development, project management, business development, patent law, management consulting, venture capital and entrepreneurs. Once matched, mentees will meet with their mentor monthly to discuss specific career-building topics. As part of the process, participants will create a personal strategic plan for identifying ways to achieve career goals.
CAN program has been successfully held for the past six years and received many positive feedbacks. It is an excellent opportunity to interact and network with a diverse group of people in academia and industry in a supportive and informal setting. 2016 CAN program will continue to provide our members an interactive relationship with mentors discussing personalized career issues such as work life balance, transition alternative career path, entrepreneurship in US and China, workplace interpersonal skills and managerial skills.
Join our kickoff event to learn more about the program details and network with mentors and other mentees. You will have the opportunity of identifying a mutually agreed-upon mentor or mentee at the meeting.
Seats are limited. The online application link and mentor list will be published CABS website after 3/26 kickoff.
Registration Requirement:
Free to members and non-members
Register at: http://goo.gl/forms/DBlBZK0Tt8
1633 Old Bayshore Hwy, Burlingame, CA 94010

EPPIC Opens Registration for EPPICon 2016
Parking is complimentary at the event and can be validated at EPPICon registration desk.
More details about this eventRegistration Link: https://www.123signup.com/register?id=ppjrk
EPPICon 2016
10th Annual Flagship Conference
3D - The Convergence of Drug Discovery, Diagnostics and Digital Health
It all begins with accurate diagnostics. Discovery of accurate biomarkers triggers the development of new therapeutic drugs as well as complementary patient side diagnostic tools. In addition, advances and miniaturization in technology is now leading to the democratization of diagnostics by placing diagnostic tools in the form of wearables and cloud-connected home devices reign the hands of end users. With the increasing emphasis on personalized medicine and targeted therapies, diagnostics and biomarkers are playing an increasingly important role in drug development.
EPPICon 2016 will focus on the convergence of these three fields and present speakers and panel discussions to spark the entire day of programming on hot topics related to development of drugs, diagnostics and digital health. EPPIC has invited thought leaders who are shaping the future of the life science industry through technological innovation. There will be time to network, jostle around ideas with like minded people, meet individuals about investment opportunities and form new alliances.
Don't miss this one of a kind content rich conference and an exquisite opportunity to help build the future of medicine!
Join us for an exciting EPPICon 2016!
When: Saturday, March 26, 2016, 8:00 am - 7:30 pm
Where: Crowne Plaza, San Francisco Airport, 1177 Airport Blvd, Burlingame, CA 94010
Who Should Attend:
Entrepreneurs, Innovators, Researchers, Investors and Professionals (including Service Providers) from Biotech, Pharma, Medical Devices, Diagnostics, Digital Health, IT, Innovative Technology and Academia
Registration: https://www.123signup.com/register?id=ppjrk
Early Bird Registration: $125 (ends on Feb 29th)
Advance Registration: $150 (March 1st - 25th)
On-site Registration: $175 (March 26th)
Early Bird Student Registration: $50 (ends February 29)**
EPPIC Charter Members* / Sponsors* / Event Speakers*
*Renew or Register: Conference is complimentary
**Student promo code "STCON16". Valid student ID to be presented at Conference
Crowne Plaza, San Francisco Airport, 1177 Airport Blvd, Burlingame, CA 94010
Agenda
1:30 - 2:00 pm Registration | Refreshment
2:00 - 3:30 pm Facilitated Speech & Discussion
3:30 - 4:00 pm Q&A
Speakers:
2:00–2:30 pm Investment Strategies during Volatility
Kai Chen, CFA
Founder & Managing Director, OceanIQ Capital
2:30–3:00 pm Taming the Bear Market
Vice President, MassMutual Northern California
Stephen Koh, CLF
3:00–3:30 pm Personal Lessons for Financial Planning
Pharmaceutical Director, ZC2 Consulting
Bill Choy, PhD
Moderator:
Frank Zhou, PhD
Registration:
CBA Alliance: Online registration: Free
Please copy and past the link:
[https://www.eventbrite.com/e/mitigating-future-financial-uncertainties-tickets-23006567262]
On-site registration: $5
Speakers Info:
Finance seminar program and brochure 201503.docx
Oshman Family Jewish Community Center Meeting Room E104, 3921 Fabian Way, Palo Alto, CA, 94303
Click here for details
The Moscone North Convention Center, 747 Howard Street, San Francisco, CA 94103
China Healthcare Investment Conference ( CHIC ) is organizing a Business Plan Competition as a pre-program of its 2016 meeting. The top three selections will be invited to present at the 7th CHIC conference ( March 29-31, 2016 at the Shangri-La Pudong Hotel in Shanghai ). Please see attached announcement for details.
How to Enter: Submit a non-confidential business plan (less than 20 pages) by sending tocompetition@chinahic.com.
Who Can Participate: Seed round to Series A round healthcare and life sciences companies
Important Deadlines:
March 1st, 2016 , 5pm China time – Deadline for business plan submissions
March 15th, 2016 , 5pm China time – Deadline of confirmation to present at CHIC by finalists
Withdrawal Penalty: If a confirmed company drops out after March 17th, 2016 , 5pm China time, the company will be ineligible to participate in next year’s competition.
Entry Fee: The CHIC business plan competition does not have an entry fee.
Use of Conference Materials: CHIC reserves the right to use any material prepared for the competition in publicizing and promoting the event.
Changes to Competition Rules: While not anticipated, the rules are subject to change at the discretion of CHIC at any time. CHIC reserves the right to interpret these rules according to its own judgment.
Website: www.chinahic.com/competition.php
WeChat: Add CHICAdmin as your WeChat friend.
Shangri-La Pudong Hotel in Shanghai, China
CABS Seminar
Topic: Financial Planning: Risk Management
Host: CABS Social Life Committee
Time: Feb 27, 2016 2:00pm-4:00pm
Location: Foster City Recreation Center, Mist Room
650 Shell Blvd, Foster City, CA 94404
Seminar introduction:
Do you know you might lose everything you have if you make one mistake? So what is your risk exposures? What is your risk tolerance or risk aversion? Have you done your risk analyst? Do you have good plan to protect your assets? Do you know what your policy covers? Do you know you might save up to thousands every year by reviewing your insurance policies?
We'll discuss:
· How do you find your risk exposures?
· Analyze your risk tolerance
· Choose the most suitable and affordable protection plan
· Risk management tools and strategies to help fine-tune your financial strategy
Registration: free
Organized by CABS Social Life Committee
About Joanna Zheng
Joanna Zheng is a certified financial planner who aims to help her customers to appreciate the value of financial planning. She has worked as an agent for farmers insurance since 1995 and obtained her security 6&63 license with farmers financial solutions in 2001. 20 years of financial industrial experience backs her advice and credentials, ensuring optimal results for her clientele. She can save you up to thousands dollars by reviewing your insurance policies and give you professional advices by analyzing your current investment portfolios. Her mission is helping her clients achieve their dreams.
Her business phone number is : 650-5251128
Website: www.zenpitney.com
E-mail: jzheng4081@yahoo.com
Foster City Recreation Center, Mist Room 650 Shell Blvd, Foster City, CA 94404
The Commonwealth Club of California & Committee of 100 Presents:
"The U.K. sees China as a friend, Why don't we?"
with Martin Jacques and Susan Shirk
and moderated by George Koo
Thursday, February 18, 2016
SAP, 3410 Hillview Avenue, Palo Alto 94303
6:00p.m. – Check In
6:30-7:30 p.m. – Program
As Great Britain enters what some are calling the “Golden Era” of relations with China, the United States continues with its tenuous and tense relationship with the Middle Kingdom. Given the historic closeness and cultural similarities between the US and the UK, what explains this tremendous difference in relations?
Understanding this question is relevant to the future of world peace and stability, and we have invited two speakers eminently qualified to shed light on this issue. Our speakers will describe the stark difference in reception of President Xi in both the US and the UK, why the UK is going its own way with Chinese relations, and the takeaways and implications arising from this issue.
Martin Jacques: Author of global best-seller When China Rules the World; Senior Fellow at the Department of Politics and International Studies, Cambridge University; Visiting Professor, Institute of Modern International Relations, Tsinghua University, Beijing; non-resident Fellow, Transatlantic Academy, Washington DC; columnist for the Guardian.
Susan Shirk: Research Professor; Chair, 21st Century China Program at the School of Global Policy and Strategy at UC San Diego, author of China: Fragile Superpower, served as Deputy Assistant Secretary of State for East Asia (1997-2000), founder of the Northeast Asia Cooperation Dialogue.
SAP, 3410 Hillview Avenue, Palo Alto 94303
Chia Tai Tianqing Pharmaceutical Group (CTTQ) is looking for talents to join. Please see attachment at the bottom for job positions.
Meanwhile, if you intend to develop and market your innovative new drugs in China, CTTQ would be your ideal partner of choice. CTTQ is interested in collaborating with overseas universities, research institutes, and pharma/biotech companies via licensing, R & D collaboration, co-invention, and strategic partnership.
If interested, please send your CV or project slide deck to tong.xu@cttq.com in order to get onsite interview opportunity. Meanwhile, everyone is welcome to attend this event to obtain information. Registration online (at the bottom) is highly recommended. We need head count to prepare food.
Chia Tai Tianqing Pharmaceutical Group (CTTQ) attendee list:
Chia Tai Tianqing Pharmaceutical Group (CTTQ) attendee list:
Mr. Jie Zhang, Vice President
Ms. Hongmei Gu, Assistant President; Vice President, R & D Institute
Dr. Ling Yang, Vice President of Project Management, R & D Institute
Dr. Yinsheng Zhang, Senior Director of Medicinal Chemistry, R & D Institute
Dr. Wei Zhao, Technical Director, R & D Institute
Dr. Yong Zhang, Head of International Regulatory Affairs, R & D Institute
Dr. Tong Xu, Chief Representative – North America
活动日程 (agenda)
6:00-6:30PM 自助晚餐,社交时间 (dinner, networking)
6:30-6:45PM CABS的PPT介绍 (CABS introduction)
6:45-6:50PM 正大天晴参会人员介绍 (CTTQ delegates’ introduction)
6:50-7:20PM 正大天晴PPT整体介绍(徐彤)(CTTQ introduction)
7:20-7:35PM 正大天晴新项目合作介绍:寻求项目特征描述,成功案例等(杨玲)(collaboration introduction, case study)
7:35-7:50PM 正大天晴海外招聘介绍(张杰,顾红梅)(recruitment introduction)
7:50-8:20PM 问答 (Q&A)
8:20-9:30PM 分成三组活动 (three groups’ activities)
项目合作 (collaboration); 杨玲,徐彤; 6个15分钟的一对一会谈
海外招聘 (recruitment); 张杰,顾红梅,赵伟; 6个15分钟的一对一会谈
信息交流 (communication on all areas); 张寅生,张勇; 覆盖所有话题
公司介绍 (The attachment below is the company introduction in English)
正大天晴药业集团股份有限公司(以下简称“正大天晴”)是中国集科研,生产和销售为一体的大型综合型医药企业,2015年营业额人民币133亿元,在2014年度“中国医药工业百强榜”上排名第20位,目前集团员工总数7500人。母公司中国生物制药有限公司(Sino Biopharmaceutical Ltd.)2004年在香港证券交易所上市(股票代码:1177.hk),目前市值超过500亿港元。
正大天晴药业集团研究院成立于1985年,在江苏南京和连云港市拥有两个研发中心,员工数超过730人,正大天晴在2015年“中国医药研发产品线最佳工业企业”排名第4位。 研究院目前积极招聘国际人才,以努力提高中国本土医药企业创新能力,及推动制剂成品走向国际主要市场。研究院同时积极寻求与海外机构的新药研发合作,争取共赢机遇。我们感兴趣的疾病领域广泛,包括癌症,肝病,心脑血管疾病,呼吸系统疾病,糖尿病,传染病,胃肠道疾病等。除新药(NME)外,其它感兴趣领域包括505(b)(2), 已上市专利药新生产工艺,新剂型项目等。
If there are any questions, please call 858-774-0534.
1633 Bayshore Hwy, Unit 280, Burlingame, CA, 94010
Seminar by Prof. Steve Quake from Stanford University.
Stephen Quake, Professor at Stanford University, inventor and entrepreneur.
Stephen Quake is an American scientist, inventor and entrepreneur. He earned his B.S. in Physics and M.S. in Mathematics from Stanford in 1991 and his D.Phil. in Physics from Oxford University in 1994 as a Marshall Scholar. His thesis research was in statistical mechanics and the effects of knots on polymers. He did his postdoctoral work at Stanford in single molecules biophysics with Steven Chu. Quake joined the faculty of the California Institute of Technology at the age of 26, where he rose through the ranks and was ultimately appointed the Thomas and Doris Everhart Professor of Applied Physics and Physics. He moved back to Stanford University in 2005 to help launch a new department in Bioengineering, where he is now the Lee Otterson Professor of Bioengineering and Applied Physics, and an Investigator of the Howard Hughes Medical Institute.
Quake has been elected to the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, the American Physical Society and the American Institute for Medical and Biological Engineering. He is the recipient of numerous international awards, including the Human Frontiers of Science Nakasone Prize, the MIT-Lemelson Prize for Innovation, the Raymond and Beverly Sackler International Prize in Biophysics, the NIH Director’s Pioneer Award, the American Society of Microbiology’s Promega Biotechnology Award, and the Royal Society of Chemistry Publishing’s Pioneer of Miniaturization Award. He has founded or co-founded several companies, including Fluidigm, Helicos Biosciences, Verinata Health, Quanticel Pharmaceuticals, Moleculo, Cellular Research and Immumetrix. Quake is a leader in experimental biophysics known for his pioneering new approaches to biological measurement. He has made many contributions to the field of microfluidics, including the invention of microfluidic large scale integration, and developed applications of microfluidics to areas as diverse as structural biology, drug discovery, and molecular affinity measurements. He has also made numerous contributions to the field of genomics, including the first single molecule DNA sequencing, techniques to perform single cell gene expression and genome sequencing, the development of non-invasive prenatal diagnostics to replace amniocentesis, prenatal genome sequencing, non-invasive tests for heart transplant rejection, and the development of approaches to sequence and analyze an individual's immune system. He was one of the first handful of people in the world to see their own genomes, and his genome was the subject of clinical annotation by a large team in the Stanford Hospital.
RegistrationFree for CABS member, but online registration is required.
Free for Stanford student and postdoc or affiliates, online registration is not required but verification of Stanford ID is required at the gate
$40 for non-member or join CABS and get this event for free!
$50 for onsite registration, CABS member or non-member.
ALWAY M106, 291 Campus Drive, Stanford, CA 94305
 QPS is a GLP/GCP-compliant contract research organization (CRO) supporting discovery, preclinical and clinical drug development. We provide quality services to pharmaceutical and biotechnology clients worldwide.
QPS is a GLP/GCP-compliant contract research organization (CRO) supporting discovery, preclinical and clinical drug development. We provide quality services to pharmaceutical and biotechnology clients worldwide.
http://www.qps-usa.com/

Established in December 2000, WuxiApptec is the leading global CRO that provides a broad and integrated portfolio of laboratory and manufacturing services across the discovery to commercialization spectrum.
http://www.wuxiapptec.com

Cosmo Biotechnologies is the first plastic system solution provider of bio-medical devices in the world,We have a lots of know-how,experience and background in this field and well organized engineering team,We base in Silicon Valley,USA and Science Industrial Park,Guangzhou,China,Our facilities under the clear room of the 10,000 class. and follow with the ISO13485 system management.
http://www.cosmobrand.com
 The mission for Genentech is to be the leading biotechnology company, using human genetic information to develop, manufacture and market pharmaceuticals that address significant unmet medical needs. We commit ourselves to high standards of integrity in contributing to the best interests of patients, the medical profession, and our employees, and to seeking significant returns to our stockholders based on the continued pursuit of excellent science.
The mission for Genentech is to be the leading biotechnology company, using human genetic information to develop, manufacture and market pharmaceuticals that address significant unmet medical needs. We commit ourselves to high standards of integrity in contributing to the best interests of patients, the medical profession, and our employees, and to seeking significant returns to our stockholders based on the continued pursuit of excellent science.
http://www.genentech.com/gene/
 Hangzhou Economic and Technological Development Area (HEDA) is a State Level development zone governing an area of 104.7 square kilometers as approved by the State Council in April 1993. Comprehensive development ranks among the top 15 among 49 national economic development zones. For 13 years, HEDA has been an important base for industrial modernization, foreign economic involvement, high education and life science research. A total of more than 400 foreign enterprises from 30 countries have settled in HEDA, bringing contracted foreign capital of US$4.21 billion and actual foreign capital of US$1.93 billion. Fifty-plus world-famous companies and multinationals are involved. Thirty-eight projects attract investment from 26 enterprises in the Fortune 500. Relying on the pillar industries of electronic information, biopharmaceuticals, home appliances, automobile parts, textiles and light foodstuffs, HEDA plows forward, becoming increasingly high-tech. Within HEDA, a college town including 15 plus universities and the Zhejing Export Processing Zone also provide adequate human resources and an excellent business environment and export/industrial infrastructure.
Hangzhou Economic and Technological Development Area (HEDA) is a State Level development zone governing an area of 104.7 square kilometers as approved by the State Council in April 1993. Comprehensive development ranks among the top 15 among 49 national economic development zones. For 13 years, HEDA has been an important base for industrial modernization, foreign economic involvement, high education and life science research. A total of more than 400 foreign enterprises from 30 countries have settled in HEDA, bringing contracted foreign capital of US$4.21 billion and actual foreign capital of US$1.93 billion. Fifty-plus world-famous companies and multinationals are involved. Thirty-eight projects attract investment from 26 enterprises in the Fortune 500. Relying on the pillar industries of electronic information, biopharmaceuticals, home appliances, automobile parts, textiles and light foodstuffs, HEDA plows forward, becoming increasingly high-tech. Within HEDA, a college town including 15 plus universities and the Zhejing Export Processing Zone also provide adequate human resources and an excellent business environment and export/industrial infrastructure.
目前HEDA正聚焦于生物医药产业,积极引进海外人才在开发区创新创业,有兴趣来HEDA的人可以联系虞先生。
湾区联系人:虞向群
Email:yuxqwork@gmail.com
电话:(408) 650-9908
HEDA 的征集链接:
http://www.hedarc.gov.cn/index/newsshow-418495503307.html
http://www.heda.gov.cn
 Founded by a group of veterans from the US biopharmaceutical industry in 2004, Sundia MediTech Company Ltd. is now a leading Shanghai-based pharmaceutical and biotech R&D out-sourcing company. Recently merged with United PharmaTech and forming an alliance with HD Biosciences, Sundia offers broad and integrated services of custom organic synthesis, medicinal chemistry, process chemistry, APIs manufacturing under cGMP or non-cGMP, bioassay, and in vitro & in vivo PK studies.
Founded by a group of veterans from the US biopharmaceutical industry in 2004, Sundia MediTech Company Ltd. is now a leading Shanghai-based pharmaceutical and biotech R&D out-sourcing company. Recently merged with United PharmaTech and forming an alliance with HD Biosciences, Sundia offers broad and integrated services of custom organic synthesis, medicinal chemistry, process chemistry, APIs manufacturing under cGMP or non-cGMP, bioassay, and in vitro & in vivo PK studies.
http://www.sundia.com/
 Optivia Biotechnology provides high throughput transporter assays to clients and partners involved with drug discovery. Optivia strives to offer more transporters in better in vitro models than any other provider. Our customers use information from our assays to discover compounds with improved bioavailability and targeted tissue distribution leading to safer, more efficacious drugs that are more likely to succeed in human clinical trial. Let the Optivia transporter technology enable you to discover better drugs.
Optivia Biotechnology provides high throughput transporter assays to clients and partners involved with drug discovery. Optivia strives to offer more transporters in better in vitro models than any other provider. Our customers use information from our assays to discover compounds with improved bioavailability and targeted tissue distribution leading to safer, more efficacious drugs that are more likely to succeed in human clinical trial. Let the Optivia transporter technology enable you to discover better drugs.
http://www.optiviabio.com/

Pharmaron is a premier R&D service provider for the pharmaceutical industry, helping clients advance their projects in a timely and cost effective manner. With a world-class team of over 900 scientists and highly experienced and accomplished management in the US and China, Pharmaron provides partners with optimal outsourcing solutions in the drug discovery area, including synthetic and high throughput library chemistry, medicinal chemistry, biology, DMPK, in vivo pharmacology, toxicology, process development and large scale manufacturing. In addition to strong stand-alone services in each area, Pharmaron also offers integrated drug discovery and development services.
http://www.pharmaron.com
 BioDuro is a leading integrated drug discovery outsourcing company that provides high-quality, integrated research and development services to biopharmaceutical clients. Our services range from medicinal chemistry to discovery biology, pharmacology, drug metabolism and pharmacokinetics (DMPK) and drug safety evaluation.
BioDuro is a leading integrated drug discovery outsourcing company that provides high-quality, integrated research and development services to biopharmaceutical clients. Our services range from medicinal chemistry to discovery biology, pharmacology, drug metabolism and pharmacokinetics (DMPK) and drug safety evaluation.
Founded in 2006, BioDuro has 675 employees, the majority of whom are based in Beijing, where the company operates a state-of-the-art, 110,000-square-foot laboratory. BioDuro has the highest concentration of drug discovery expertise of any contract research organization (CRO) in China.
In November 2009, BioDuro joined PPD, a leading global CRO providing drug discovery, development and life cycle management services. Together, PPD and BioDuro perform a broad range of research and development services for large pharmaceutical clients from early stage through Phase IV.
http://www.bioduro.com/
 Contact Singapore is an agency of the Singapore government whose primary function is to draw people from around the world to work, invest and live in Singapore, with the ultimate aim of boosting economic development. It is an alliance of Singapore's Economic Development Board and the Ministry of Manpower.
Contact Singapore is an agency of the Singapore government whose primary function is to draw people from around the world to work, invest and live in Singapore, with the ultimate aim of boosting economic development. It is an alliance of Singapore's Economic Development Board and the Ministry of Manpower.
http://www.contactsingapore.sg

Medicilon is a fully integrated drug discovery service company that can help clients from idea stage to US IND filing stage.
http://www.mediciloninc.com/

淄博高新技术产业开发区位于山东省中部,是1992年11月国务院批准设立的国家级高新区,辖区总面积121平方公里。淄博高新区被先后认定为国家新材料、先进陶瓷、功能玻璃、生物医药、创新药物孵化等特色产业基地,正在建设国家创新型科技园区、知识产权试点园区,在129个国家高新区中创新能力评价位居30位。淄博高新区正逐步成为鲁中地区高新技术产业发展的示范区,自主创新引领区、科技创业的核心区。近年来,投资30余亿元,建成了一个综合孵化器、生物医药、精细化工和高分子、先进陶瓷、电子信息、先进制造等五个专业孵化器,淄博瀚海硅谷科技园、淄博瀚海慕尼黑科技园、台北齐鲁科技园三个海外孵化器,总面积达110万平方米,其中国家级孵化器5个;投资3亿多元高标准建设了MEMS研发中试、生物医药、精细化工和高分子材料、无机非金属材料、电子信息五大公共技术服务平台,创新平台建设走在全国高新区前列。各类孵化器共入驻企业及机构502家,入驻率达80%以上,五大技术平台为1400余家企业提供25000余次检测服务。
生物医药产业是淄博高新区重点发展的新兴产业,依托生物医药公共技术服务平台,高新区管委会与山东大学合作建设了山东大学淄博生物医药研究院,下设药物分析测试中心、技术资料信息查询中心、生物药物实验室、化学药物实验室、天然药物实验室、药物制剂实验室等六个功能单元,拥有大中型仪器设备487台(套),仪器设备总投资4600余万元,已建立起完善的生物医药“技术研发服务链”与“成果转化链”。在区域经济发展中,承担着医药科技成果转移与转化、公共技术服务、实用型技术人才培育、科技交流与合作等多方面职能。同时,高新区企业在消毒灭菌设备、放射治疗系统、制药装备等医疗器械和解热镇痛药、头孢类抗生素、中药等新医药领域保持国内领先地位,拥有新华制药、新华医疗、德国贝朗、中化帝斯曼、金城柯瑞、世博金 都等一批骨干企业。
http://www.china-zibo.gov.cn/

The old year leaves amidst the falling snow
瑞雪纷飞辞旧岁
The new spring comes with the shining glow
旭日东升迎新春
Happy Chinese New Year!!! Please join us on Sunday, January 31, 2016, to kick off the Year of the Monkey with exciting CABS traditional New Year Festivities!
11:30 AM - 1:00 PM: Chinese New Year Lunch
1:00 PM - 4:00 PM: CABS Chinese New Year Gala
Program to include: Singing and dancing; Beijing opera; Live musical entertainments; Spring festival couplets and conundrum, and much more!
There will be games for children and lucky draws for everybody! Many prize for you and the Top One is an Apple iPad mini!!! Come to reconnect with old friends, make new ones and have fun! Let’s celebrate Chinese New Year 2016 together!
We look forward to seeing you there!

Registration Requirement:
On-Line:
Member: $10; Non-Member: $15
On-Site:
Member: $15; Non-Member: $20
Children < 12 years of age are free and
Seniors > 70 years old will be $5.
Special thanks to our sponsors (click for details):
Eureka Therapeutics Inc (Gavin Lu, Gold Sponsor)
Berkshire Hathaway CA Realty (Andy Gan, Gold Sponsor)
Financial Guide Gold (Rong Shen, Gold Sponsor)
New York Life (Winny Lin, Silver Sponsor)
Zenpitney LLC (Joanna Zheng, Silver Sponsor)
William E. Walker Recreation Center, 650 Shell Boulevard, Foster City, CA
For details see below:
网上注册early bird免费。现场注册20美金
Follow the link below to register
Online registration
Stanford University LKSC Center Berg Hall
CABS K. Fong Award in Life Sciences is presented annually to recognize those individuals who make significant contributions in life sciences and biopharmaceutical industry including outstanding scientific findings, recognized efforts in promoting life science education and initiatives in improving life science community, and those who bring therapeutic breakthroughs to the market and improve healthcare and quality of life.
Candidates must be nominated by an active member of CABS. Selection criteria will be based on candidate’s accomplishments in life sciences and contribution to the life science community, including one or all of the following:
- Proven achievements in therapeutic breakthroughs (including discovery, process or clinical development), diagnostics or the research reagent/equipment markets.
- Significant contribution to the promotion of academic and industrial R&D in biomedical sciences and applications.
- Significant contribution to the CABS community and to promoting international collaboration in life sciences.
Winners will be selected by the CABS Award Selection Committee. The winner(s) will receive a $2,500 award and a Plaque of Merit at the Annual CABS Conference (BioPacific Conference) on May 7th, 2016.
About Dr. Kenneth Fong:
Dr. Kenneth Fong is a successful entrepreneur, an investor and mentor to many biotech founders, a community leader supporting higher education and a philanthropist. Currently Dr. Fong is the Chairman of Kenson Ventures, LLC, a venture capital firm that specializes in venture financing and strategic consulting to biotech companies. He is the founder and former CEO of Clontech, which developed and marketed Molecular Biology products to academic and pharmaceutical laboratories in the US and thirty other countries. He sits on the Boards of numerous biotech companies and serves on the advisory board of biotech-related entities in the US, China, Taiwan and Hong Kong.
Dr. Fong is active with both philanthropic and community activities. For example, he and his wife were major contributors in establishing the Fong Optometry and Medical Library at UC Berkeley, the Fong Scholarship Programs / Annual Scientific Symposia at San Francisco State University, the Annual Fong-Clontech Seminar series at Indiana University. His Community services included Chairman of the San Jose Chinese Performing Arts Center (2000-02), lead supporter to the Chinese Historical Society of America (SF, 2000) and the pioneering Biotech Program at the San Jose Tech Museum (1999). Recently, he has become more supportive of the educational programs for children in under-served and impoverished regions of China. He is a member of Committee 100 and a former member of the Board of 80-20 Initiative.
Online
For more visit us: http://2016sv.pmwcintl.com
Follow us on Twitter: @PMWCintl
Registration Requirement:
Computer History Museum 1401 N SHORELINE BLVD MOUNTAIN VIEW, CA 94043
From this seminar, you will gain a better understanding of this disease, which is your first step to fully embracing self-care. Dr. Lin will teach you some tips to help you develop an effective self-care plan.
About Dr. Lin
Dr. Ling was raised in a family of doctors, having started practice in 1992. Prior to coming to US, she worked as an attending physician for over 6 years at Peking Union Medical College Hospital in Beijing, the best hospital in China and accumulated much experience from the world-known experts in different fields. Dr. Ling is a board-certified physician in US, Primary Care Physician. She finished USMLE exams with scores of 99% and finished her residency in New York. Before start of her own clinic in Burlingame, she worked as a staff physician at Asian Health Services in Oakland, CA. In addition to her medical training, Dr. Ling received her Ph.D in Pharmaceutical Sciences with GPA of 4.0 and received Pfizer Recognition Award and Johnson & Johnson Standards of Leadership Award, for her work on Clinical Trials in the fields of diabetes, oncology, etc. Her expertise in drug mechanisms and drug-drug interactions means that she can provide patients with the best personalized care, and the best medicine combination with minimal side effects. Even with years of experience, she always takes her time to give the best care for each individual patient. Her clinic address: 1828 El Camino Real #405, Burlingame, CA 94010. Her website: DrJennyLing.com
Registration Requirement: Free
Organizers: CABS Social Life Committee and Silicon Valley Sichuan University Alumni Association (SVSUAA)
Foster City Recreation Center, Mist Room 650 Shell Blvd, Foster City, CA 94404
As drug researchers, we were often excited about the “great” drug candidates and “great” drug targets we discovered, but only found out later that side effects (off-target toxicity) could not be practically handled, or the concern was too big. We wished if only we could deliver the drug to some site, or avoid some area. ADC (antibody drug conjugates) has generated exciting news, which is only a small part of targeted drug delivery. This workshop will present not only the advancement in ADC, but also other exciting progress and benefits of drug delivery to drug discovery and development.
Agenda:
| 9:30 - 10:00 AM | Registration, Coffee and Networking |
| 10:00 - 10:10 AM | Opening remarks and introduction |
| 10:10 - 11:00 AM | The Marriage of Nanomedicines with Cell Therapies; a Practical Approach or a Means to Keep Academic Drug Delivery Scientists Gainfully Employed? |
| Francis C. Szoka, Ph.D., Professor of Bioengineering, Therapeutic Science & Pharmaceutical Chemistry at the University of California School of Pharmacy in San Francisco | |
| 11:00 - 11:45 AM | Chemically Modified Viral Capsids as Versatile Nanoscale Delivery Agents |
| Matthew B. Francis, Ph.D., Professor of Chemistry, University of California, Berkeley, and Materials Sciences Division, Lawrence Berkeley National Laboratory | |
| 11:45 AM - 1:00 PM | Lunch & Networking |
| 1:00 - 1:45 PM | A New Biocompatible Condensation Reaction and Its Application for Molecular Imaging and Drug Delivery |
| Jianghong Rao, Ph.D., Associate Professor of Radiology and Chemistry (courtesy), as well as a member of the Molecular Imaging, Bio-X, Cancer Biology, and Biophysics Programs at Stanford University School of Medicine | |
| 1:45 - 2:30 PM | XTEN™ - Protein Polymer as a Drug Delivery Platform |
| Vladimir N. Podust, Ph.D., Director of Analytical Chemistry, Amunix Operating Inc. | |
| 2:30 - 3:15 PM | Stable and Efficacious Site Specific ADCs of Un-modified Antibodies Made Easy by Engineered Transglutaminase |
| Sean Hu, Ph.D., Senior Vice President, Dophen BioMed, Inc. | |
| 3:15 - 4:30 PM | Closing remarks & Networking |
* Lunch will be provided.
Registration Requirement:
Online registration is strongly encouraged.
Online: $25 for CABS members, $35 for non-members
Onsite: $45
Embassy Suites, 250 Gateway Blvd., South San Francisco, CA 94080
Panelists
Nisa Leung, Managing Partner, Qiming Venture
Masood Tayebi, Investor, Bridgewest Business Group
Mike Liu, Head Global BD, Hengrui Pharma
Wei Zheng, Co-founder, Suzhou Connect
Janet Xiao, Partner, Morrison & Foerster
Greg Scott, Founder, ChinaBio® Group (Moderator)
Agenda:
8:30 - 9:00 am Breakfast and networking
9:00 - 10:30 am Panel discussion
10:30 - 11:00 am Networking
This is an invitation only event. Please register early to secure your seat. Registration fee is complimentary.
Click Here to Register
Organizers: Chinese American BioPharmaceutical Society; ChinaBio; Morrison & Foerster
Morrison & Foerster LLP, 425 Market Street, San Francisco, CA 94105-2482
Introduction of Tianjin Asymchem Company and 1:1 onsite interview (Dinner will be provided)
 The Company Introduction
The Company Introduction Asymchem Laboratories Inc.
- One of the world’s leading contract manufacturing organizations providing the pharmaceutical industry with APIs and API intermediates
- Customers include 13 of the world’s 15 largest pharmaceutical companies plus many emerging pharma companies
- Sustained growth rate exceeding 25% annually
- IPO planned
- A research-intensive company actively developing new methodology for more effective processes
- The company strives to deliver innovative processes at every stage of your new product pipeline, providing practical and cost-effective scale-ups from route development, through pilot scale to launch.
- Please see the attached company introduction file for more details including the benefits.
- Asymchem is providing a boundless platform for global top talents to make an impact to the whole world! If you are ready to impact the world together with Asymchem, join us!
- 2016 Asymchem Overseas Campus Recruiting aims at 2016 graduates as well as people with graduate degree and past working experiences in the US (San Francisco, Boston, San Diego)
- The job openings focus on the talents in chemistry field and include Researchers and Sr. Researchers positions. People with master degree could be project leaders and people with doctor degree could be core team leaders.
- Please see the details in the job description. Please also see the attached event poster (You are welcome to scan the two-dimension code on poster to follow the update).
| How to apply for the position: | If you are interested in the position, please email your resume to liangyin@asymchem.com.cn AND fill out the registration form below. |
| How to just attend the meeting: | If you are not ready to submit resume but interested in attending the meeting to hear more about the company, please fill out the registration form below. |
| If you are not available on Dec. 15: | If you cannot attend the Dec. 15 meeting, we will schedule sometime on Dec. 16 to meet you! Please fill out the registration form below. |
| Registration Requirement | Registration is free. Click for registration form. |
| Organizers | CABS International Collaboration Committee, Business & Career Development Committee, and Membership Committee |
UC Berkeley Conference Room (The address is TBD. We will send an update email to the registrants.)




%20(1).svg)


















.JPG)









































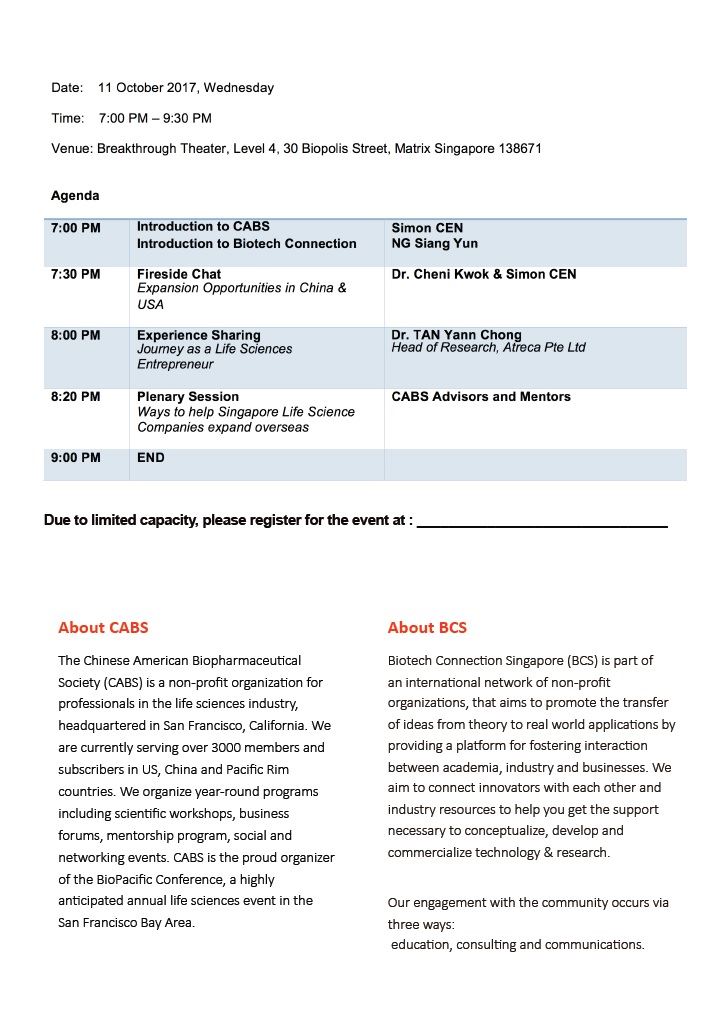


 As crazy as this may sound, Life Science Nation (LSN) has more early stage investors signed up for
As crazy as this may sound, Life Science Nation (LSN) has more early stage investors signed up for 












